The 3Rs in Experimental Liver Disease
- PMID: 37508134
- PMCID: PMC10376896
- DOI: 10.3390/ani13142357
The 3Rs in Experimental Liver Disease
Abstract
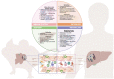
Patients with cirrhosis present multiple physiological and immunological alterations that play a very important role in the development of clinically relevant secondary complications to the disease. Experimentation in animal models is essential to understand the pathogenesis of human diseases and, considering the high prevalence of liver disease worldwide, to understand the pathophysiology of disease progression and the molecular pathways involved, due to the complexity of the liver as an organ and its relationship with the rest of the organism. However, today there is a growing awareness about the sensitivity and suffering of animals, causing opposition to animal research among a minority in society and some scientists, but also about the attention to the welfare of laboratory animals since this has been built into regulations in most nations that conduct animal research. In 1959, Russell and Burch published the book "The Principles of Humane Experimental Technique", proposing that in those experiments where animals were necessary, everything possible should be done to try to replace them with non-sentient alternatives, to reduce to a minimum their number, and to refine experiments that are essential so that they caused the least amount of pain and distress. In this review, a comprehensive summary of the most widely used techniques to replace, reduce, and refine in experimental liver research is offered, to assess the advantages and weaknesses of available experimental liver disease models for researchers who are planning to perform animal studies in the near future.
Keywords: disease; liver; reduction; refinement; replacement; research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Replacement, reduction and refinement.ALTEX. 2002;19(2):73-8. ALTEX. 2002. PMID: 12098013
-
60 Years of the 3Rs symposium: Lessons learned and the road ahead.ALTEX. 2024;41(2):179-201. doi: 10.14573/altex.2403061. ALTEX. 2024. PMID: 38629803
-
Culture of Care: Organizational Responsibilities.In: Weichbrod RH, Thompson GA, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. In: Weichbrod RH, Thompson GA, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. PMID: 29787190 Free Books & Documents. Review.
-
Practical Application of the 3Rs in Rodent Transgenesis.Methods Mol Biol. 2023;2631:33-51. doi: 10.1007/978-1-0716-2990-1_2. Methods Mol Biol. 2023. PMID: 36995663
-
Conceptual foundations for a clarified meaning of the 3Rs principles in animal experimentation.Anim Welf. 2024 Sep 23;33:e37. doi: 10.1017/awf.2024.39. eCollection 2024. Anim Welf. 2024. PMID: 39347486 Free PMC article. Review.
Cited by
-
Green tea and hyaluronic acid gel enhance fibroblast activation and improves the gingival healing post-third molar extraction.Sci Rep. 2024 Mar 26;14(1):7124. doi: 10.1038/s41598-024-57821-5. Sci Rep. 2024. PMID: 38531928 Free PMC article. Clinical Trial.
-
Animal model considerations for chordoma research: reproducing the tumor microenvironment in vivo with humanized mice.Front Oncol. 2024 Mar 13;14:1330254. doi: 10.3389/fonc.2024.1330254. eCollection 2024. Front Oncol. 2024. PMID: 38544830 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

