Modulation of Cell Response through the Covalent Binding of Fibronectin to Titanium Substrates
- PMID: 37504837
- PMCID: PMC10381834
- DOI: 10.3390/jfb14070342
Modulation of Cell Response through the Covalent Binding of Fibronectin to Titanium Substrates
Abstract
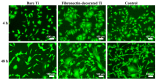
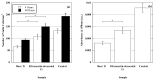
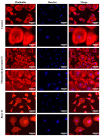
Titanium (Ti-6Al-4V) substrates were functionalized through the covalent binding of fibronectin, and the effect of the existence of this extracellular matrix protein on the surface of the material was assessed by employing mesenchymal stem cell (MSC) cultures. The functionalization process comprised the usage of the activation vapor silanization (AVS) technique to deposit a thin film with a high surface density of amine groups on the material, followed by the covalent binding of fibronectin to the amine groups using the N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS) crosslinking chemistry. The biological effect of the fibronectin on murine MSCs was assessed in vitro. It was found that functionalized samples not only showed enhanced initial cell adhesion compared with bare titanium, but also a three-fold increase in the cell area, reaching values comparable to those found on the polystyrene controls. These results provide compelling evidence of the potential to modulate the response of the organism to an implant through the covalent binding of extracellular matrix proteins on the prosthesis.
Keywords: activated vapor silanization (AVS); biomaterial; fibronectin; functionalization; mesenchymal stem cells (MSC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Improved cell adhesion to activated vapor silanization-biofunctionalized Ti-6Al-4V surfaces with ECM-derived oligopeptides.Biomater Adv. 2022 Feb;133:112614. doi: 10.1016/j.msec.2021.112614. Epub 2021 Dec 22. Biomater Adv. 2022. PMID: 35527152
-
Enhanced Biological Response of AVS-Functionalized Ti-6Al-4V Alloy through Covalent Immobilization of Collagen.Sci Rep. 2018 Feb 20;8(1):3337. doi: 10.1038/s41598-018-21685-3. Sci Rep. 2018. PMID: 29463865 Free PMC article.
-
Dental Implants with Anti-Biofilm Properties: A Pilot Study for Developing a New Sericin-Based Coating.Materials (Basel). 2019 Jul 30;12(15):2429. doi: 10.3390/ma12152429. Materials (Basel). 2019. PMID: 31366076 Free PMC article.
-
Application of single cell force spectroscopy (SCFS) to the assessment of cell adhesion to peptide-decorated surfaces.Int J Biol Macromol. 2023 Jul 31;244:125369. doi: 10.1016/j.ijbiomac.2023.125369. Epub 2023 Jun 14. Int J Biol Macromol. 2023. PMID: 37321435
-
Design and biological functionality of a novel hybrid Ti-6Al-4V/hydrogel system for reconstruction of bone defects.J Tissue Eng Regen Med. 2018 Apr;12(4):1133-1144. doi: 10.1002/term.2614. Epub 2017 Dec 10. J Tissue Eng Regen Med. 2018. PMID: 29134773
Cited by
-
Modification of 316L Stainless Steel, Nickel Titanium, and Cobalt Chromium Surfaces by Irreversible Immobilization of Fibronectin: Towards Improving the Coronary Stent Biocompatibility.Molecules. 2024 Oct 18;29(20):4927. doi: 10.3390/molecules29204927. Molecules. 2024. PMID: 39459295 Free PMC article.
References
-
- Brunette D.M., Tengvall P., Textor M., Thomsen P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2012.
-
- Liu X., Chu P.K., Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004;47:49–121. doi: 10.1016/j.mser.2004.11.001. - DOI
-
- Chen Q., Thouas G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015;87:1–57. doi: 10.1016/j.mser.2014.10.001. - DOI
-
- Geetha M., Singh A.K., Asokamani R., Gogia A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009;54:397–425. doi: 10.1016/j.pmatsci.2008.06.004. - DOI
-
- Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E. Biomaterials Science: An Introduction to Materials in Medicine. Elsevier Science; Amsterdam, The Netherlands: 2004.
Grants and funding
LinkOut - more resources
Full Text Sources

