Balance beam crossing times are slower after noise exposure in rats
- PMID: 37497526
- PMCID: PMC10368468
- DOI: 10.3389/fnint.2023.1196477
Balance beam crossing times are slower after noise exposure in rats
Abstract
Introduction: The vestibular system integrates signals related to vision, head position, gravity, motion, and body position to provide stability during motion through the environment. Disruption in any of these systems can reduce agility and lead to changes in ability to safely navigate one's environment. Causes of vestibular decline are diverse; however, excessive noise exposure can lead to otolith organ dysfunction. Specifically, 120 decibel (dB) sound pressure level (SPL) 1.5 kHz-centered 3-octave band noise (1.5 kHz 3OBN) causes peripheral vestibular dysfunction in rats, measured by vestibular short-latency evoked potential (VsEP) and reduced calretinin-immunolabeling of calyx-only afferent terminals in the striolar region of the saccule. The present study examined the functional impact of this noise exposure condition, examining changes in motor performance after noise exposure with a balance beam crossing task.
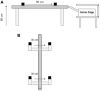
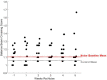
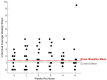
Methods: Balance beam crossing time in rats was assessed for 19 weeks before and 5 weeks after noise exposure. Balance beam crossings were scored to assess proficiency in the task. When animals were proficient, they received a single exposure to 120 dB SPL 3-octave band noise.
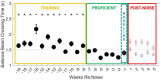
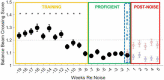
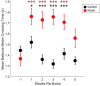
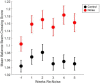
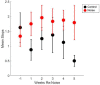
Results: During the initial training phase slower crossing times and higher scores, including multiple failures were observed. This was followed by a period of significant improvement leading to proficiency, characterized by fast and stable crossing times and consistently low scores. After noise exposure, crossing times were significantly elevated from baseline for 4-weeks. A total of 5 weeks after noise exposure, crossing times improved, and though still trending higher than baseline, they were no longer significantly different from baseline.
Discussion: These findings show that the noise-induced peripheral vestibular changes we previously observed at cellular and electro-physiological levels also have an impact at a functional level. It has been previously shown that imbalance is associated with slower walking speed in older adults and aged rats. These findings in noise-exposed rats may have implications for people who experience noisy environments and for seniors with a history of noise exposure who also experience balance disorders and may be at increased fall risk.
Keywords: balance; mobility; motor impairment; noise exposure; noise-induced vestibular loss; vestibular.
Copyright © 2023 Bartikofsky, Hertz, Bauer, Altschuler, King and Stewart.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Intense noise exposure alters peripheral vestibular structures and physiology.J Neurophysiol. 2020 Feb 1;123(2):658-669. doi: 10.1152/jn.00642.2019. Epub 2019 Dec 25. J Neurophysiol. 2020. PMID: 31875485 Free PMC article.
-
Transient peripheral vestibular hypofunction measured with vestibular short-latency evoked potentials following noise exposure in rats.J Neurophysiol. 2021 Nov 1;126(5):1547-1554. doi: 10.1152/jn.00131.2021. Epub 2021 Sep 22. J Neurophysiol. 2021. PMID: 34550030 Free PMC article.
-
Vestibular short-latency evoked potential abolished by low-frequency noise exposure in rats.J Neurophysiol. 2018 Feb 1;119(2):662-667. doi: 10.1152/jn.00668.2017. Epub 2017 Nov 8. J Neurophysiol. 2018. PMID: 29118200 Free PMC article.
-
Effects of Noise Exposure on the Vestibular System: A Systematic Review.Front Neurol. 2020 Nov 25;11:593919. doi: 10.3389/fneur.2020.593919. eCollection 2020. Front Neurol. 2020. PMID: 33324332 Free PMC article. Review.
-
Peripheral vestibular system: Age-related vestibular loss and associated deficits.J Otol. 2021 Oct;16(4):258-265. doi: 10.1016/j.joto.2021.06.001. Epub 2021 Jun 11. J Otol. 2021. PMID: 34548873 Free PMC article. Review.
Cited by
-
Andaliman (Zanthoxylum acanthopodium DC.) fruit ethanolic extract exerts attenuative effect on hyperglycemia, sensory and motoric function's disorders in alloxan-induced diabetic mice.J Adv Vet Anim Res. 2023 Dec 31;10(4):608-619. doi: 10.5455/javar.2023.j716. eCollection 2023 Dec. J Adv Vet Anim Res. 2023. PMID: 38370902 Free PMC article.
-
Rapid ejaculator rats are more susceptible to anxiety compared with normal ejaculator rats.Int J Impot Res. 2024 Apr 15. doi: 10.1038/s41443-024-00888-5. Online ahead of print. Int J Impot Res. 2024. PMID: 38622269
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

