Neural activity induces strongly coupled electro-chemo-mechanical interactions and fluid flow in astrocyte networks and extracellular space-A computational study
- PMID: 37478153
- PMCID: PMC10396022
- DOI: 10.1371/journal.pcbi.1010996
Neural activity induces strongly coupled electro-chemo-mechanical interactions and fluid flow in astrocyte networks and extracellular space-A computational study
Abstract
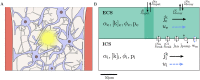
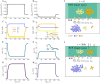
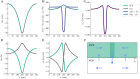
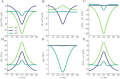
The complex interplay between chemical, electrical, and mechanical factors is fundamental to the function and homeostasis of the brain, but the effect of electrochemical gradients on brain interstitial fluid flow, solute transport, and clearance remains poorly quantified. Here, via in-silico experiments based on biophysical modeling, we estimate water movement across astrocyte cell membranes, within astrocyte networks, and within the extracellular space (ECS) induced by neuronal activity, and quantify the relative role of different forces (osmotic, hydrostatic, and electrical) on transport and fluid flow under such conditions. We find that neuronal activity alone may induce intracellular fluid velocities in astrocyte networks of up to 14μm/min, and fluid velocities in the ECS of similar magnitude. These velocities are dominated by an osmotic contribution in the intracellular compartment; without it, the estimated fluid velocities drop by a factor of ×34-45. Further, the compartmental fluid flow has a pronounced effect on transport: advection accelerates ionic transport within astrocytic networks by a factor of ×1-5 compared to diffusion alone.
Copyright: © 2023 Sætra et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Aquaporin-4-dependent K(+) and water transport modeled in brain extracellular space following neuroexcitation.J Gen Physiol. 2013 Jan;141(1):119-32. doi: 10.1085/jgp.201210883. J Gen Physiol. 2013. PMID: 23277478 Free PMC article.
-
Spatial model of convective solute transport in brain extracellular space does not support a "glymphatic" mechanism.J Gen Physiol. 2016 Dec;148(6):489-501. doi: 10.1085/jgp.201611684. Epub 2016 Nov 11. J Gen Physiol. 2016. PMID: 27836940 Free PMC article.
-
Theoretical analysis of wake/sleep changes in brain solute transport suggests a flow of interstitial fluid.Fluids Barriers CNS. 2022 Apr 13;19(1):30. doi: 10.1186/s12987-022-00325-z. Fluids Barriers CNS. 2022. PMID: 35418142 Free PMC article.
-
The role of brain barriers in fluid movement in the CNS: is there a 'glymphatic' system?Acta Neuropathol. 2018 Mar;135(3):387-407. doi: 10.1007/s00401-018-1812-4. Epub 2018 Feb 10. Acta Neuropathol. 2018. PMID: 29428972 Review.
-
Glial diffusion barriers during aging and pathological states.Prog Brain Res. 2001;132:339-63. doi: 10.1016/S0079-6123(01)32087-3. Prog Brain Res. 2001. PMID: 11545002 Review.
Cited by
-
The role of astrocytes in the glymphatic network: a narrative review.Metab Brain Dis. 2024 Mar;39(3):453-465. doi: 10.1007/s11011-023-01327-y. Epub 2023 Nov 27. Metab Brain Dis. 2024. PMID: 38008886 Review.
-
An electrodiffusive network model with multicompartmental neurons and synaptic connections.PLoS Comput Biol. 2024 Nov 12;20(11):e1012114. doi: 10.1371/journal.pcbi.1012114. eCollection 2024 Nov. PLoS Comput Biol. 2024. PMID: 39531480 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

