An adaptive behavioral control motif mediated by cortical axo-axonic inhibition
- PMID: 37474640
- PMCID: PMC10400431
- DOI: 10.1038/s41593-023-01380-x
An adaptive behavioral control motif mediated by cortical axo-axonic inhibition
Abstract
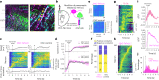
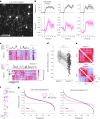
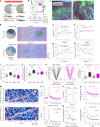
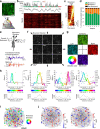
Genetically defined subgroups of inhibitory interneurons are thought to play distinct roles in learning, but heterogeneity within these subgroups has limited our understanding of the scope and nature of their specific contributions. Here we reveal that the chandelier cell (ChC), an interneuron type that specializes in inhibiting the axon-initial segment (AIS) of pyramidal neurons, establishes cortical microcircuits for organizing neural coding through selective axo-axonic synaptic plasticity. We found that organized motor control is mediated by enhanced population coding of direction-tuned premotor neurons, with tuning refined through suppression of irrelevant neuronal activity. ChCs contribute to learning-dependent refinements by providing selective inhibitory control over individual pyramidal neurons rather than global suppression. Quantitative analysis of structural plasticity across axo-axonic synapses revealed that ChCs redistributed inhibitory weights to individual pyramidal neurons during learning. These results demonstrate an adaptive logic of the inhibitory circuit motif responsible for organizing distributed neural representations. Thus, ChCs permit efficient cortical computation in a targeted cell-specific manner.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Update of
-
An adaptive behavioral control motif mediated by cortical axo-axonic inhibition.bioRxiv [Preprint]. 2023 Mar 10:2023.03.10.531767. doi: 10.1101/2023.03.10.531767. bioRxiv. 2023. Update in: Nat Neurosci. 2023 Aug;26(8):1379-1393. doi: 10.1038/s41593-023-01380-x. PMID: 36945592 Free PMC article. Updated. Preprint.
Similar articles
-
An adaptive behavioral control motif mediated by cortical axo-axonic inhibition.bioRxiv [Preprint]. 2023 Mar 10:2023.03.10.531767. doi: 10.1101/2023.03.10.531767. bioRxiv. 2023. Update in: Nat Neurosci. 2023 Aug;26(8):1379-1393. doi: 10.1038/s41593-023-01380-x. PMID: 36945592 Free PMC article. Updated. Preprint.
-
Activity-Dependent Plasticity of Axo-axonic Synapses at the Axon Initial Segment.Neuron. 2020 Apr 22;106(2):265-276.e6. doi: 10.1016/j.neuron.2020.01.037. Epub 2020 Feb 27. Neuron. 2020. PMID: 32109363 Free PMC article.
-
Specific and comprehensive genetic targeting reveals brain-wide distribution and synaptic input patterns of GABAergic axo-axonic interneurons.Elife. 2024 Jul 16;13:RP93481. doi: 10.7554/eLife.93481. Elife. 2024. PMID: 39012795 Free PMC article.
-
Lighting the chandelier: new vistas for axo-axonic cells.Trends Neurosci. 2005 Jun;28(6):310-6. doi: 10.1016/j.tins.2005.04.004. Trends Neurosci. 2005. PMID: 15927687 Review.
-
Chandelier Cells in Functional and Dysfunctional Neural Circuits.Front Neural Circuits. 2016 May 4;10:33. doi: 10.3389/fncir.2016.00033. eCollection 2016. Front Neural Circuits. 2016. PMID: 27199673 Free PMC article. Review.
Cited by
-
Experience shapes chandelier cell function and structure in the visual cortex.Elife. 2024 Jan 9;12:RP91153. doi: 10.7554/eLife.91153. Elife. 2024. PMID: 38192196 Free PMC article.
-
Axo-axonic synaptic input drives homeostatic plasticity by tuning the axon initial segment structurally and functionally.Sci Adv. 2024 Aug 2;10(31):eadk4331. doi: 10.1126/sciadv.adk4331. Epub 2024 Aug 2. Sci Adv. 2024. PMID: 39093969 Free PMC article.
-
Specific and Plastic: Chandelier Cell-to-Axon Initial Segment Connections in Shaping Functional Cortical Network.Neurosci Bull. 2024 Nov;40(11):1774-1788. doi: 10.1007/s12264-024-01266-3. Epub 2024 Jul 30. Neurosci Bull. 2024. PMID: 39080101 Free PMC article. Review.
-
Axo-axonic cells in neuropsychiatric disorders: a systematic review.Front Cell Neurosci. 2023 Jun 26;17:1212202. doi: 10.3389/fncel.2023.1212202. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37435048 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

