Role(s) of G3BPs in Human Pathogenesis
- PMID: 37468286
- PMCID: PMC10519580
- DOI: 10.1124/jpet.122.001538
Role(s) of G3BPs in Human Pathogenesis
Abstract
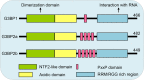
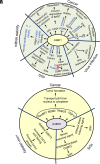
Ras-GTPase-activating protein (SH3 domain)-binding proteins (G3BP) are RNA binding proteins that play a critical role in stress granule (SG) formation. SGs protect critical mRNAs from various environmental stress conditions by regulating mRNA stability and translation to maintain regulated gene expression. Recent evidence suggests that G3BPs can also regulate mRNA expression through interactions with RNA outside of SGs. G3BPs have been associated with a number of disease states, including cancer progression, invasion, metastasis, and viral infections, and may be useful as a cancer therapeutic target. This review summarizes the biology of G3BP including their structure, function, localization, role in cancer progression, virus replication, mRNA stability, and SG formation. We will also discuss the potential of G3BPs as a therapeutic target. SIGNIFICANCE STATEMENT: This review will discuss the molecular mechanism(s) and functional role(s) of Ras-GTPase-activating protein (SH3 domain)-binding proteins in the context of stress granule formation, interaction with viruses, stability of RNA, and tumorigenesis.
Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
Similar articles
-
Rasputin a decade on and more promiscuous than ever? A review of G3BPs.Biochim Biophys Acta Mol Cell Res. 2019 Mar;1866(3):360-370. doi: 10.1016/j.bbamcr.2018.09.001. Epub 2018 Sep 5. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30595162 Free PMC article. Review.
-
Role of Chikungunya nsP3 in Regulating G3BP1 Activity, Stress Granule Formation and Drug Efficacy.Arch Med Res. 2021 Jan;52(1):48-57. doi: 10.1016/j.arcmed.2020.10.002. Epub 2020 Oct 31. Arch Med Res. 2021. PMID: 33131924
-
Research Progress on the Structure and Function of G3BP.Front Immunol. 2021 Aug 30;12:718548. doi: 10.3389/fimmu.2021.718548. eCollection 2021. Front Immunol. 2021. PMID: 34526993 Free PMC article. Review.
-
G3BP1 and G3BP2 regulate translation of interferon-stimulated genes: IFITM1, IFITM2 and IFITM3 in the cancer cell line MCF7.Mol Cell Biochem. 2019 Sep;459(1-2):189-204. doi: 10.1007/s11010-019-03562-3. Epub 2019 Jun 6. Mol Cell Biochem. 2019. PMID: 31172368
-
Stress granule components G3BP1 and G3BP2 play a proviral role early in Chikungunya virus replication.J Virol. 2015 Apr;89(8):4457-69. doi: 10.1128/JVI.03612-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653451 Free PMC article.
Cited by
-
Protein-protein interactions with G3BPs drive stress granule condensation and gene expression changes under cellular stress.bioRxiv [Preprint]. 2024 Feb 7:2024.02.06.579149. doi: 10.1101/2024.02.06.579149. bioRxiv. 2024. PMID: 38370785 Free PMC article. Preprint.
References
-
- Ashikari D, Takayama K, Tanaka T, Suzuki Y, Obinata D, Fujimura T, Urano T, Takahashi S, Inoue S (2017) Androgen induces G3BP2 and SUMO-mediated p53 nuclear export in prostate cancer. Oncogene 36:6272–6281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
