Prediction of herpes virus infections after solid organ transplantation: a prospective study of immune function
- PMID: 37465673
- PMCID: PMC10351284
- DOI: 10.3389/fimmu.2023.1183703
Prediction of herpes virus infections after solid organ transplantation: a prospective study of immune function
Abstract
Introduction: Herpes virus infections are a major concern after solid organ transplantation and linked to the immune function of the recipient. We aimed to determine the incidence of positive herpes virus (cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus type 1/2 (HSV-1/2), and varicella zoster virus (VZV)) PCR tests during the first year post-transplantation and assess whether a model including immune function pre-transplantation and three months post-transplantation could predict a subsequent positive herpes virus PCR.
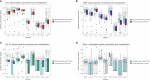
Methods: All participants were preemptively screened for CMV, and EBV IgG-negative participants were screened for EBV during the first year post-transplantation. Herpes virus PCR tests for all included herpes viruses (CMV, EBV, HSV-1/2, and VZV) were retrieved from the Danish Microbiology database containing nationwide PCR results from both hospitals and outpatient clinics. Immune function was assessed by whole blood stimulation with A) LPS, B) R848, C) Poly I:C, and D) a blank control. Cytokine concentrations (TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12p40, IL-17A, IFN-α, and IFN-γ) were measured using Luminex.
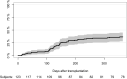
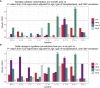
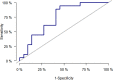
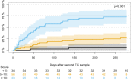
Results: We included 123 liver (54%), kidney (26%), and lung (20%) transplant recipients. The cumulative incidence of positive herpes virus PCR tests was 36.6% (95% CI: 28.1-45.1) during the first year post-transplantation. The final prediction model included recipient age, type of transplantation, CMV serostatus, and change in Poly I:C-induced IL-12p40 from pre-transplantation to three months post-transplantation. The prediction model had an AUC of 77% (95% CI: 61-92). Risk scores were extracted from the prediction model, and the participants were divided into three risk groups. Participants with a risk score <5 (28% of the cohort), 5-10 (45% of the cohort), and >10 (27% of the cohort) had a cumulative incidence of having a positive herpes virus PCR test at 5.8%, 25%, and 73%, respectively (p < 0.001).
Conclusion: In conclusion, the incidence of positive herpes virus PCR tests was high, and a risk model including immune function allowed the prediction of positive herpes virus PCR and may be used to identify recipients at higher risk.
Keywords: TruCulture®; cytomegalovirus; herpes virus; immune functional assay; prediction; solid organ transplantation.
Copyright © 2023 Møller, Sørensen, Rezahosseini, Rasmussen, Arentoft, Loft, Perch, Gustafsson, Lundgren, Scheike, Knudsen, Ostrowski, Rasmussen and Nielsen.
Conflict of interest statement
OR received a grant from the Research Foundation of Rigshospitalet related to this work, and a grant from AP Møller Fonden not related to this work. MP has participated in advisory boards for Takeda, and PulmonX, served as a research consultant for AMBU, received an unlimited institutional research grant from Roche and travel grants from Boeringer-Ingelheim, received lecturing honorary from AstraZeneca, GSK, Therakos and PulmonX but declares no conflict of interest directly related to the study; FG reports consulting fees from Pfizer, Alnylam, Ionis, and Abbott and lecture fees from Novartis, not related to this work; SN received unrestricted research grants from Novo Nordisk Foundation and Independent Research Fund FSS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Seroprevalence of cytomegalovirus in donors & opportunistic viral infections in liver transplant recipients.Indian J Med Res. 2017 Apr;145(4):558-562. doi: 10.4103/ijmr.IJMR_1024_14. Indian J Med Res. 2017. PMID: 28862190 Free PMC article.
-
Infections caused by herpes viruses other than cytomegalovirus in solid organ transplant recipients.Enferm Infecc Microbiol Clin. 2012 Mar;30 Suppl 2:63-9. doi: 10.1016/S0213-005X(12)70084-8. Enferm Infecc Microbiol Clin. 2012. PMID: 22542037 Review.
-
Preventive Strategies Against Cytomegalovirus and Incidence of α-Herpesvirus Infections in Solid Organ Transplant Recipients: A Nationwide Cohort Study.Am J Transplant. 2017 Jul;17(7):1813-1822. doi: 10.1111/ajt.14192. Epub 2017 Feb 2. Am J Transplant. 2017. PMID: 28039960
-
Four-Parameter FluoroSpot Assay Reveals That the Varicella Zoster Virus Elicits a Robust Memory T Cell IL-10 Response throughout Childhood.J Virol. 2022 Nov 23;96(22):e0131022. doi: 10.1128/jvi.01310-22. Epub 2022 Oct 31. J Virol. 2022. PMID: 36314824 Free PMC article.
-
Herpes Virus Infection in Lung Transplantation: Diagnosis, Treatment and Prevention Strategies.Viruses. 2023 Nov 27;15(12):2326. doi: 10.3390/v15122326. Viruses. 2023. PMID: 38140567 Free PMC article. Review.

