Mechanism and therapeutic implications of pomalidomide-induced immune surface marker upregulation in EBV-positive lymphomas
- PMID: 37463943
- PMCID: PMC10354044
- DOI: 10.1038/s41598-023-38156-z
Mechanism and therapeutic implications of pomalidomide-induced immune surface marker upregulation in EBV-positive lymphomas
Abstract
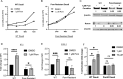
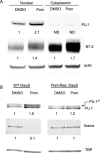
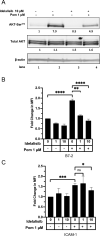
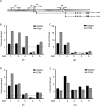
Epstein-Barr virus (EBV) downregulates immune surface markers to avoid immune recognition. Pomalidomide (Pom) was previously shown to increase immune surface marker expression in EBV-infected tumor cells. We explored the mechanism by which Pom leads to these effects in EBV-infected cells. Pom increased B7-2/CD86 mRNA, protein, and surface expression in EBV-infected cells but this was virtually eliminated in EBV-infected cells made resistant to Pom-induced cytostatic effects. This indicates that Pom initiates the upregulation of these markers by interacting with its target, cereblon. Interestingly, Pom increased the proinflammatory cytokines IP-10 and MIP-1∝/β in EBV infected cells, supporting a possible role for the phosphoinositide 3-kinase (PI3K)/AKT pathway in Pom's effects. Idelalisib, an inhibitor of the delta subunit of PI3 Kinase, blocked AKT-Ser phosphorylation and Pom-induced B7-2 surface expression. PU.1 is a downstream target for AKT that is expressed in EBV-infected cells. Pom treatment led to an increase in PU.1 binding to the B7-2 promoter based on ChIP analysis. Thus, our data indicates Pom acts through cereblon leading to degradation of Ikaros and activation of the PI3K/AKT/PU.1 pathway resulting in upregulation of B7-2 mRNA and protein expression. The increased immune recognition in addition to the increases in proinflammatory cytokines upon Pom treatment suggests Pom may be useful in the treatment of EBV-positive lymphomas.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Drs. Yarchoan and Davis are co-inventors on US Patent 10,001,483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV induced lymphoma using immunomodulatory compounds, and uses of biomarkers”. In is our understanding that foreign patents have also been filed for this invention. An immediate family member of R. Yarchoan is a co-inventor on patents or patent applications related to internalization of target receptors, epigenetic analysis, and ephrin tyrosine kinase inhibitors. These inventions were all made as full-time employees of the US government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to these patents have been or should by law be assigned to the U.S. Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99–502). This research was supported in part by a CRADA between the NCI and Celgene Corporation (now Bristol Myers Squibb). Dr. Yarchoan also reports receiving drugs for clinical trials from Merck, EMD-Serano, Eli Lilly, and CTI BioPharma through CRADAs with the NCI, and he has received drug supply for laboratory research from Janssen Pharmaceuticals.
Figures


Similar articles
-
Pomalidomide increases immune surface marker expression and immune recognition of oncovirus-infected cells.Oncoimmunology. 2018 Dec 5;8(2):e1546544. doi: 10.1080/2162402X.2018.1546544. eCollection 2019. Oncoimmunology. 2018. PMID: 30713808 Free PMC article.
-
Syk activation of phosphatidylinositol 3-kinase/Akt prevents HtrA2-dependent loss of X-linked inhibitor of apoptosis protein (XIAP) to promote survival of Epstein-Barr virus+ (EBV+) B cell lymphomas.J Biol Chem. 2011 Oct 28;286(43):37368-78. doi: 10.1074/jbc.M111.255125. Epub 2011 Sep 9. J Biol Chem. 2011. PMID: 21908615 Free PMC article.
-
Pomalidomide restores immune recognition of primary effusion lymphoma through upregulation of ICAM-1 and B7-2.PLoS Pathog. 2021 Jan 7;17(1):e1009091. doi: 10.1371/journal.ppat.1009091. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33411730 Free PMC article.
-
Herpesviruses in the Activated Phosphatidylinositol-3-Kinase-δ Syndrome.Front Immunol. 2018 Feb 23;9:237. doi: 10.3389/fimmu.2018.00237. eCollection 2018. Front Immunol. 2018. PMID: 29599765 Free PMC article. Review.
-
Epstein--Barr virus post-transplant lymphoproliferative disease and virus-specific therapy: pharmacological re-activation of viral target genes with arginine butyrate.Transpl Infect Dis. 2001 Sep;3(3):177-85. doi: 10.1034/j.1399-3062.2001.003003177.x. Transpl Infect Dis. 2001. PMID: 11493400 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

