The malaria blood stage antigen PfCyRPA formulated with the TLR-4 agonist adjuvant GLA-SE elicits parasite growth inhibitory antibodies in experimental animals
- PMID: 37454145
- PMCID: PMC10349406
- DOI: 10.1186/s12936-023-04638-8
The malaria blood stage antigen PfCyRPA formulated with the TLR-4 agonist adjuvant GLA-SE elicits parasite growth inhibitory antibodies in experimental animals
Abstract
Background: Plasmodium falciparum cysteine-rich protective antigen (PfCyRPA) is an invasion complex protein essential for erythrocyte invasion. In contrast to several previously clinically tested merozoite vaccine candidate antigens, PfCyRPA is not polymorphic, making it a promising candidate antigen for blood stage vaccine development.
Methods: Mice and rabbits were immunized with vaccine formulations of recombinantly expressed PfCyRPA adjuvanted either with the glucopyranosyl lipid A (GLA) containing adjuvants GLA-LSQ, GLA-SE, GLA-Alum or with Nanoalum. ELISA and indirect immunofluorescence assays (IFA) were used to analyse elicited IgG titers and the P. falciparum growth inhibitory activity was determined with a standardized in vitro [3H]-hypoxanthine incorporation assay.
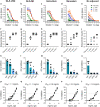
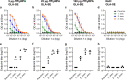
Results: In the mouse experiments, the GLA adjuvanted formulations were superior to the Nanoalum formulation with respect to antibody titer development, IFA sero-conversion rates and in vitro parasite growth-inhibitory activity. In rabbits, the highest titers of parasite growth inhibitory antibodies were obtained with the GLA-SE formulation. Comparable mean ELISA IgG endpoint titers were reached in rabbits after three immunizations with GLA-SE adjuvanted PfCyRPA doses of 5, 25 and 100 µg, but with 100 µg of antigen, only two immunizations were required to reach this titer.
Conclusion: PfCyRPA formulated with the human-compatible adjuvant GLA-SE represents an attractive vaccine candidate for early clinical testing in a controlled P. falciparum blood stage challenge trial.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Generation of Plasmodium falciparum parasite-inhibitory antibodies by immunization with recombinantly-expressed CyRPA.Malar J. 2016 Mar 15;15:161. doi: 10.1186/s12936-016-1213-x. Malar J. 2016. PMID: 26979066 Free PMC article.
-
IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice.PLoS One. 2014 Oct 24;9(10):e111020. doi: 10.1371/journal.pone.0111020. eCollection 2014. PLoS One. 2014. PMID: 25343487 Free PMC article.
-
Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® in European and African adults: A phase 1a/1b, randomized, double-blind multi-centre trial.Vaccine. 2017 Oct 27;35(45):6218-6227. doi: 10.1016/j.vaccine.2017.09.027. Epub 2017 Sep 22. Vaccine. 2017. PMID: 28947345 Clinical Trial.
-
Identification of adjuvants for clinical trials performed with Plasmodium falciparum AMA1 in rabbits.BMC Immunol. 2019 Jul 30;20(1):25. doi: 10.1186/s12865-019-0307-y. BMC Immunol. 2019. PMID: 31362695 Free PMC article.
-
A novel asexual blood-stage malaria vaccine candidate: PfRipr5 formulated with human-use adjuvants induces potent growth inhibitory antibodies.Front Immunol. 2022 Oct 27;13:1002430. doi: 10.3389/fimmu.2022.1002430. eCollection 2022. Front Immunol. 2022. PMID: 36389677 Free PMC article.
Cited by
-
Vaccines and monoclonal antibodies: new tools for malaria control.Clin Microbiol Rev. 2024 Jun 13;37(2):e0007123. doi: 10.1128/cmr.00071-23. Epub 2024 Apr 24. Clin Microbiol Rev. 2024. PMID: 38656211 Review.
-
Development of an improved blood-stage malaria vaccine targeting the essential RH5-CyRPA-RIPR invasion complex.Nat Commun. 2024 Jun 7;15(1):4857. doi: 10.1038/s41467-024-48721-3. Nat Commun. 2024. PMID: 38849365 Free PMC article.
-
The Need for Novel Asexual Blood-Stage Malaria Vaccine Candidates for Plasmodium falciparum.Biomolecules. 2024 Jan 12;14(1):100. doi: 10.3390/biom14010100. Biomolecules. 2024. PMID: 38254700 Free PMC article. Review.
References
-
- RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

