Evidence-based clinical practice guidelines for cholelithiasis 2021
- PMID: 37452855
- PMCID: PMC10423145
- DOI: 10.1007/s00535-023-02014-6
Evidence-based clinical practice guidelines for cholelithiasis 2021
Abstract
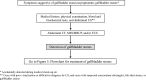
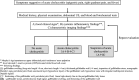
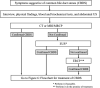
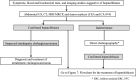
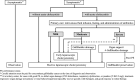
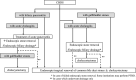
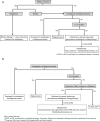
The Japanese Society of Gastroenterology first published evidence-based clinical practice guidelines for cholelithiasis in 2010, followed by a revision in 2016. Currently, the revised third edition was published to reflect recent evidence on the diagnosis, treatment, and prognosis of cholelithiasis conforming to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. Following this revision, the present English version of the guidelines was updated and published herein. The clinical questions (CQ) in the previous version were reviewed and rearranged into three newly divided categories: background questions (BQ) dealing with basic background knowledge, CQ, and future research questions (FRQ), which refer to issues that require further accumulation of evidence. Finally, 52 questions (29 BQs, 19 CQs, and 4 FRQs) were adopted to cover the epidemiology, pathogenesis, diagnosis, treatment, complications, and prognosis. Based on a literature search using MEDLINE, Cochrane Library, and Igaku Chuo Zasshi databases for the period between 1983 and August 2019, along with a manual search of new information reported over the past 5 years, the level of evidence was evaluated for each CQ. The strengths of recommendations were determined using the Delphi method by the committee members considering the body of evidence, including benefits and harms, patient preference, and cost-benefit balance. A comprehensive flowchart was prepared for the diagnosis and treatment of gallbladder stones, common bile duct stones, and intrahepatic stones, respectively. The current revised guidelines are expected to be of great assistance to gastroenterologists and general physicians in making decisions on contemporary clinical management for cholelithiasis patients.
Keywords: Cholelithiasis; Common bile duct stones; Endoscopic treatment; Gallbladder stones; Intrahepatic stones; Surgical treatment.
© 2023. The Author(s).
Conflict of interest statement
Any financial relationship with enterprises, businesses or academic institutions in the subject matter or materials discussed in the manuscript are listed as follows: (1) those from which the authors, the spouse, partner or immediate relatives of the authors have received individually any income, honoraria or any other type of renumeration; Asahi Kasei Pharma, Olympus Corporation, Daiichi Sankyo, Fuji Film, Taiho Pharmaceutical, Denka, and (2) those from which the authors have received scholarship/research grant; Taiho Pharmaceutical, Tsumura & Co., Bristol Myers Squibb, Ajimonoto, Hitachi, Boston Scientific Japan, Denka, Hospital Administration Niigata Prefecture, Brourbon, Yasuda Yogurt, Astellas Pharmaceutical, Abbvie, EA Pharma, Eisai, MSD, Ono Pharmaceutical, Covidien Japan, Taiho Pharmaceutical, Takeda Pharmaceutical, Chugai Pharmaceutical, Novaltis Japan, Bayer Japan, Asahi Kasei Pharma, Nippon Kayaku, Mochida Pharmaceutical, Eli Lilly Japan, Gadelius Medical, Boston Scientific Japan, JRA Facilities, and (3) those from which the academic institutions of the authors received support (commercial/academic cooperation); Eisai.
Figures
Comment in
-
Understanding evidence-based clinical practice guidelines for cholelithiasis 2021.Hepatobiliary Surg Nutr. 2024 Apr 3;13(2):352-355. doi: 10.21037/hbsn-24-25. Epub 2024 Mar 27. Hepatobiliary Surg Nutr. 2024. PMID: 38617501 Free PMC article. No abstract available.
Similar articles
-
Evidence-based clinical practice guidelines for cholelithiasis 2016.J Gastroenterol. 2017 Mar;52(3):276-300. doi: 10.1007/s00535-016-1289-7. Epub 2016 Dec 10. J Gastroenterol. 2017. PMID: 27942871 Review.
-
Evidence-based clinical practice guidelines for Liver Cirrhosis 2020.J Gastroenterol. 2021 Jul;56(7):593-619. doi: 10.1007/s00535-021-01788-x. Epub 2021 Jul 7. J Gastroenterol. 2021. PMID: 34231046 Free PMC article. Review.
-
Evidence-based clinical practice guidelines for liver cirrhosis 2020.Hepatol Res. 2021 Jul;51(7):725-749. doi: 10.1111/hepr.13678. Epub 2021 Jul 6. Hepatol Res. 2021. PMID: 34228859
-
Evidence-based clinical practice guidelines for inflammatory bowel disease 2020.J Gastroenterol. 2021 Jun;56(6):489-526. doi: 10.1007/s00535-021-01784-1. Epub 2021 Apr 22. J Gastroenterol. 2021. PMID: 33885977 Free PMC article. Review.
-
Evidence-based clinical practice guidelines for inflammatory bowel disease.J Gastroenterol. 2018 Mar;53(3):305-353. doi: 10.1007/s00535-018-1439-1. Epub 2018 Feb 10. J Gastroenterol. 2018. PMID: 29429045 Free PMC article.
Cited by
-
Characterizing the relationships between dietary indices, gallstone prevalence and the need for gallbladder surgery in the general US population.Front Nutr. 2024 May 7;11:1392960. doi: 10.3389/fnut.2024.1392960. eCollection 2024. Front Nutr. 2024. PMID: 38779446 Free PMC article.
-
Effect of the extrahepatic bile duct anatomy on choledocholithiasis and its clinical significance.World J Gastrointest Surg. 2024 May 27;16(5):1363-1370. doi: 10.4240/wjgs.v16.i5.1363. World J Gastrointest Surg. 2024. PMID: 38817273 Free PMC article.
-
Association between plant-based dietary index and gallstone disease: A cross sectional study from NHANES.PLoS One. 2024 Jun 25;19(6):e0305822. doi: 10.1371/journal.pone.0305822. eCollection 2024. PLoS One. 2024. PMID: 38917153 Free PMC article.
-
A Surgeon's Challenge: Diagnosing and Managing Hidden Bile Duct Stones Post-cholecystectomy.Cureus. 2024 Mar 25;16(3):e56865. doi: 10.7759/cureus.56865. eCollection 2024 Mar. Cureus. 2024. PMID: 38659541 Free PMC article.
-
Laparoscopic common bile duct exploration is an effective, safe, and less-costly method of treating choledocholithiasis.Surg Endosc. 2024 Oct;38(10):6076-6082. doi: 10.1007/s00464-024-11139-5. Epub 2024 Aug 13. Surg Endosc. 2024. PMID: 39138682
References
-
- Minds Manual Developing Committee. Minds Manual for Guideline Development 2017. Tokyo: Japan Council for Quality Health Care; 2017. (in Japanese)
-
- Health Statistics Association of Japan. Estimated number of patients based on patient surveys. Index of Health. 1993;39:29–35 (in Japanese).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

