PROTEIN PHOSPHATASE 2C08, a Negative Regulator of Abscisic Acid Signaling, Promotes Internode Elongation in Rice
- PMID: 37445999
- PMCID: PMC10341765
- DOI: 10.3390/ijms241310821
PROTEIN PHOSPHATASE 2C08, a Negative Regulator of Abscisic Acid Signaling, Promotes Internode Elongation in Rice
Abstract
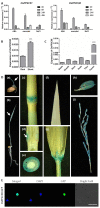
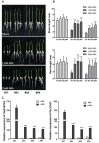
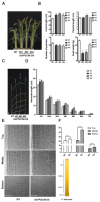
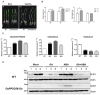
Clade A protein phosphatase 2Cs (PP2CAs) negatively regulate abscisic acid (ABA) signaling. Here, we investigated the functions of OsPP2CAs and their crosstalk with ABA and gibberellic acid (GA) signaling pathways in rice (Oryza sativa). Among the nine OsPP2CAs, OsPP2C08 had the highest amino acid sequence similarity with OsPP2C51, which positively regulates GA signaling in rice seed germination. However, OsPP2C08 was expressed in different tissues (internodes, sheaths, and flowers) compared to OsPP2C51, which was specifically expressed in seeds, and showed much stronger induction under abiotic stress than OsPP2C51. Transgenic rice lines overexpressing OsPP2C08 (OsPP2C08-OX) had a typical ABA-insensitive phenotype in a post-germination assay, indicating that OsPP2C08, as with other OsPP2CAs, negatively regulates ABA signaling. Furthermore, OsPP2C08-OX lines had longer stems than wild-type (WT) plants due to longer internodes, especially between the second and third nodes. Internode cells were also longer in OsPP2C08-OX lines than in the WT. As GA positively regulates plant growth, these results suggest that OsPP2C08 might positively regulate GA biosynthesis. Indeed, the expression levels of GA biosynthetic genes including gibberellin 20-oxidase (OsGA20ox4) and Ent-kaurenoic acid oxidase (OsKAO) were increased in OsPP2C08-OX lines, and we observed that GIBBERELLIN 2-OXIDASE 4 (OsGA2ox4), encoding an oxidase that catalyzes the 2-beta-hydroxylation of several biologically active GAs, was repressed in the OsPP2C08-OX lines based on a transcriptome deep sequencing and RT-qPCR analysis. Furthermore, we compared the accumulation of SLENDER RICE 1 (SLR1), a DELLA protein involved in GA signaling, in OsPP2C08-OX and WT plants, and observed lower levels of SLR1 in the OsPP2C08-OX lines than in the WT. Taken together, our results reveal that OsPP2C08 negatively regulates ABA signaling and positively regulates GA signaling in rice. Our study provides valuable insight into the molecular mechanisms underlying the crosstalk between GA and ABA signaling in rice.
Keywords: ABA signaling; GA biosynthesis; GA signaling; PP2CA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The protein phosphatase 2C clade A protein OsPP2C51 positively regulates seed germination by directly inactivating OsbZIP10.Plant Mol Biol. 2017 Mar;93(4-5):389-401. doi: 10.1007/s11103-016-0568-2. Epub 2016 Dec 20. Plant Mol Biol. 2017. PMID: 28000033
-
OsMFT1 Inhibits Seed Germination by Modulating Abscisic Acid Signaling and Gibberellin Biosynthesis under Salt Stress in Rice.Plant Cell Physiol. 2023 Jun 15;64(6):674-685. doi: 10.1093/pcp/pcad029. Plant Cell Physiol. 2023. PMID: 37022148
-
The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice.PLoS Genet. 2010 Sep 9;6(9):e1001098. doi: 10.1371/journal.pgen.1001098. PLoS Genet. 2010. PMID: 20838584 Free PMC article.
-
ABSCISIC ACID INSENSITIVE 5 mediates light-ABA/gibberellin crosstalk networks during seed germination.J Exp Bot. 2022 Aug 11;73(14):4674-4682. doi: 10.1093/jxb/erac200. J Exp Bot. 2022. PMID: 35522989 Review.
-
Molecular Aspects of Seed Development Controlled by Gibberellins and Abscisic Acids.Int J Mol Sci. 2022 Feb 7;23(3):1876. doi: 10.3390/ijms23031876. Int J Mol Sci. 2022. PMID: 35163798 Free PMC article. Review.
References
-
- González-García M.P., Rodríguez D., Nicolás C., Rodríguez P.L., Nicolás G., Lorenzo O. Negative regulation of abscisic acid signaling by the Fagus sylvatica FsPP2C1 plays a role in seed dormancy regulation and promotion of seed germination. Plant Physiol. 2003;133:135–144. doi: 10.1104/pp.103.025569. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

