Preclinical Assessment of ADAM9-Responsive Mesoporous Silica Nanoparticles for the Treatment of Pancreatic Cancer
- PMID: 37445886
- PMCID: PMC10341742
- DOI: 10.3390/ijms241310704
Preclinical Assessment of ADAM9-Responsive Mesoporous Silica Nanoparticles for the Treatment of Pancreatic Cancer
Abstract
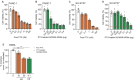
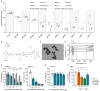
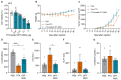
Pancreatic adenocarcinoma (PDAC) remains largely refractory to chemotherapeutic treatment regimens and, consequently, has the worst survival rate of all cancers. The low efficacy of current treatments results largely from toxicity-dependent dose limitations and premature cessation of therapy. Recently, targeted delivery approaches that may reduce off-target toxicities have been developed. In this paper, we present a preclinical evaluation of a PDAC-specific drug delivery system based on mesoporous silica nanoparticles (MSNs) functionalized with a protease linker that is specifically cleaved by PDAC cells. Our previous work demonstrated that ADAM9 is a PDAC-enriched protease and that paclitaxel-loaded ADAM9-responsive MSNs effectively kill PDAC cells in vitro. Here, we show that paclitaxel-loaded ADAM9-MSNs result in off-target cytotoxicity in clinically relevant models, which spurred the development of optimized ADAM9-responsive MSNs (OPT-MSNs). We found that these OPT-MSNs still efficiently kill PDAC cells but, as opposed to free paclitaxel, do not induce death in neuronal or bone marrow cells. In line with these in vitro data, paclitaxel-loaded OPT-MSNs showed reduced organ damage and leukopenia in a preclinical PDAC xenograft model. However, no antitumor response was observed upon OPT-MSN administration in vivo. The poor in vivo antitumor activity of OPT-MSNs despite efficient antitumor effects in vitro highlights that although MSN-based tumor-targeting strategies may hold therapeutic potential, clinical translation does not seem as straightforward as anticipated.
Keywords: MSN; PDAC; antitumor; drug delivery; leukopenia; neurotoxicity; targeted therapy.
Conflict of interest statement
M.F.B. has received research funding from Celgene, Lead Pharma, and Frame Therapeutics and acted as a consultant to Servier and Olympus. Neither of these organizations were involved in the design of this study nor the drafting of the manuscript.
Figures

Similar articles
-
CAPN2-responsive mesoporous silica nanoparticles: A promising nanocarrier for targeted therapy of pancreatic cancer.Cancer Lett. 2024 May 28;590:216845. doi: 10.1016/j.canlet.2024.216845. Epub 2024 Apr 6. Cancer Lett. 2024. PMID: 38589004
-
ADAM9-Responsive Mesoporous Silica Nanoparticles for Targeted Drug Delivery in Pancreatic Cancer.Cancers (Basel). 2021 Jul 1;13(13):3321. doi: 10.3390/cancers13133321. Cancers (Basel). 2021. PMID: 34282781 Free PMC article.
-
Functionalized Mesoporous Silica Nanoparticles for Drug-Delivery to Multidrug-Resistant Cancer Cells.Int J Nanomedicine. 2022 Jul 14;17:3079-3096. doi: 10.2147/IJN.S363952. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35859731 Free PMC article.
-
Mesoporous Silica Nanoparticle-Based Drug Delivery Systems for the Treatment of Pancreatic Cancer: A Systematic Literature Overview.Pharmaceutics. 2022 Feb 10;14(2):390. doi: 10.3390/pharmaceutics14020390. Pharmaceutics. 2022. PMID: 35214121 Free PMC article. Review.
-
Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: an update.Expert Opin Drug Deliv. 2019 Apr;16(4):415-439. doi: 10.1080/17425247.2019.1598375. Epub 2019 Apr 22. Expert Opin Drug Deliv. 2019. PMID: 30897978 Free PMC article. Review.
Cited by
-
Identification of pancreatic cancer-specific protease substrates for protease-dependent targeted delivery.Oncogenesis. 2024 Nov 20;13(1):40. doi: 10.1038/s41389-024-00542-1. Oncogenesis. 2024. PMID: 39567504 Free PMC article.
References
-
- McBride A., Bonafede M., Cai Q., Princic N., Tran O., Pelletier C., Parisi M., Patel M. Comparison of treatment patterns and economic outcomes among metastatic pancreatic cancer patients initiated on nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Expert. Rev. Clin. Pharmacol. 2017;10:1153–1160. doi: 10.1080/17512433.2017.1365598. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

