Systemic Review of Clot Retraction Modulators
- PMID: 37445780
- PMCID: PMC10341984
- DOI: 10.3390/ijms241310602
Systemic Review of Clot Retraction Modulators
Abstract
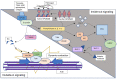
Through a process termed clot retraction, platelets cause thrombi to shrink and become more stable. After platelets are activated via inside-out signaling, glycoprotein αIIbβIII binds to fibrinogen and initiates a cascade of intracellular signaling that ends in actin remodeling, which causes the platelet to change its shape. Clot retraction is also important for wound healing. Although the detailed molecular biology of clot retraction is only partially understood, various substances and physiological conditions modulate clot retraction. In this review, we describe some of the current literature pertaining to clot retraction modulators. In addition, we discuss compounds from Cudrania trucuspidata, Arctium lappa, and Panax ginseng that diminish clot retraction and have numerous other health benefits. Caffeic acid and diindolylmethane, both common in plants and vegetables, likewise reduce clot retraction, as do all-trans retinoic acid (a vitamin A derivative), two MAP4K inhibitors, and the chemotherapeutic drug Dasatinib. Conversely, the endogenous anticoagulant Protein S (PS) and the matricellular protein secreted modular calcium-binding protein 1 (SMOC1) both enhance clot retraction. Most studies aiming to identify mechanisms of clot retraction modulators have focused on the increased phosphorylation of vasodilator-stimulated phosphoprotein and inositol 1,4,5-triphosphate receptor I and the decreased phosphorylation of various phospholipases (e.g., phospholipase A2 (PLA2) and phosphatidylinositol-specific phospholipase Cγ2 (PLCγ2), c-Jun N-terminal kinase, and (PI3Ks). One study focused on the decreased phosphorylation of Sarcoma Family Kinases (SFK), and others have focused on increased cAMP levels and the downregulation of inflammatory markers such as thromboxanes, including thromboxane A2 (TXA2) and thromboxane B2 (TXB2); prostaglandin A2 (PGE2); reactive oxygen species (ROS); and cyclooxygenase (COX) enzyme activity. Additionally, pregnancy, fibrinolysis, and the autoimmune condition systemic lupus erythematosus all seem to affect, or at least have some relation with, clot retraction. All the clot retraction modulators need in-depth study to explain these effects.
Keywords: Protein S; actin remodeling; clot retraction; platelet activation; retraction modulators; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Morin hydrate inhibits platelet activation and clot retraction by regulating integrin αIIbβ3, TXA2, and cAMP levels.Eur J Pharmacol. 2019 Dec 15;865:172734. doi: 10.1016/j.ejphar.2019.172734. Epub 2019 Oct 12. Eur J Pharmacol. 2019. PMID: 31614139
-
The antithrombotic effect of caffeic acid is associated with a cAMP-dependent pathway and clot retraction in human platelets.Thromb Res. 2020 Nov;195:87-94. doi: 10.1016/j.thromres.2020.07.024. Epub 2020 Jul 10. Thromb Res. 2020. PMID: 32682003
-
Involvement of Src kinases and PLCgamma2 in clot retraction.Thromb Res. 2007;120(2):251-8. doi: 10.1016/j.thromres.2006.09.003. Epub 2006 Oct 19. Thromb Res. 2007. PMID: 17055557 Free PMC article.
-
Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases.J Ginseng Res. 2020 Jan;44(1):24-32. doi: 10.1016/j.jgr.2019.05.005. Epub 2019 May 21. J Ginseng Res. 2020. PMID: 32095094 Free PMC article. Review.
-
Platelet signal transduction pathways: could we organize them into a 'hierarchy'?Haemostasis. 1999 Sep;29(1):4-15. doi: 10.1159/000022456. Haemostasis. 1999. PMID: 10494030 Review.
References
-
- Singh M., Akkaya S., Preuss M., Rademacher F., Tohidnezhad M., Kubo Y., Behrendt P., Weitkamp J.T., Wedel T., Lucius R., et al. Platelet-Released Growth Factors Influence Wound Healing-Associated Genes in Human Keratinocytes and Ex Vivo Skin Explants. Int. J. Mol. Sci. 2022;23:2827. doi: 10.3390/ijms23052827. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

