Interferon-expressing oncolytic adenovirus + chemoradiation inhibited pancreatic cancer growth in a hamster model
- PMID: 37439437
- PMCID: PMC10475772
- DOI: 10.1111/cas.15903
Interferon-expressing oncolytic adenovirus + chemoradiation inhibited pancreatic cancer growth in a hamster model
Abstract
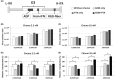
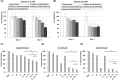
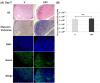
Past clinical trials of adjuvant therapy combined with interferon (IFN) alpha, fluorouracil, cisplatin, and radiation improved the 5-year survival rate of pancreatic ductal adenocarcinoma (PDAC). However, these trials also revealed the disadvantages of the systemic toxicity of IFN and insufficient delivery of IFN. To improve efficacy and tolerability, we have developed an oncolytic adenovirus-expressing IFN (IFN-OAd). Here, we evaluated IFN-OAd in combination with chemotherapy (gemcitabine + nab-paclitaxel) + radiation. Combination index (CI) analysis showed that IFN-OAd + chemotherapy + radiation was synergistic (CI <1). Notably, IFN-OAd + chemotherapy + radiation remarkably suppressed tumor growth and induced a higher number of tumor-infiltrating lymphocytes without severe side toxic effects in an immunocompetent and adenovirus replication-permissive hamster PDAC model. This is the first study to report that gemcitabine + nab-paclitaxel, the current first-line chemotherapy for PDAC, did not hamper virus replication in a replication-permissive immunocompetent model. IFN-OAd has the potential to overcome the barriers to clinical application of IFN-based therapy through its tumor-specific expression of IFN, induction of antitumor immunity, and sensitization with chemoradiation. Combining IFN-OAd with gemcitabine + nab-paclitaxel + radiation might be an effective and clinically beneficial treatment for PDAC patients.
Keywords: interferon alpha; nab-PTX; oncolytic adenovirus; oncolytic virus; pancreatic cancer.
© 2023 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no commercial associations that might be a conflict of interest in relation to this article.
Figures


Similar articles
-
Combination of interferon-expressing oncolytic adenovirus with chemotherapy and radiation is highly synergistic in hamster model of pancreatic cancer.Oncotarget. 2018 Apr 6;9(26):18041-18052. doi: 10.18632/oncotarget.24710. eCollection 2018 Apr 6. Oncotarget. 2018. PMID: 29719589 Free PMC article.
-
A NOTCH-sensitive uPAR-regulated oncolytic adenovirus effectively suppresses pancreatic tumor growth and triggers synergistic anticancer effects with gemcitabine and nab-paclitaxel.Oncotarget. 2017 Apr 4;8(14):22700-22715. doi: 10.18632/oncotarget.15169. Oncotarget. 2017. PMID: 28186974 Free PMC article.
-
An oncolytic adenovirus coding for a variant interleukin 2 cytokine improves response to chemotherapy through enhancement of effector lymphocyte cytotoxicity, fibroblast compartment modulation and mitotic slippage.Front Immunol. 2023 Jul 5;14:1171083. doi: 10.3389/fimmu.2023.1171083. eCollection 2023. Front Immunol. 2023. PMID: 37475863 Free PMC article.
-
Current Standard and Future Perspectives in First- and Second-Line Treatment of Metastatic Pancreatic Adenocarcinoma.Digestion. 2016;94(1):44-9. doi: 10.1159/000447739. Epub 2016 Jul 21. Digestion. 2016. PMID: 27438590 Review.
-
Advances in oncolytic adenovirus therapy for pancreatic cancer.Cancer Lett. 2018 Oct 10;434:56-69. doi: 10.1016/j.canlet.2018.07.006. Epub 2018 Jul 5. Cancer Lett. 2018. PMID: 29981812 Review.
Cited by
-
The investigation of oncolytic viruses in the field of cancer therapy.Front Oncol. 2024 Jul 10;14:1423143. doi: 10.3389/fonc.2024.1423143. eCollection 2024. Front Oncol. 2024. PMID: 39055561 Free PMC article. Review.
-
Oncolytic activity of a coxsackievirus B3 strain in patient-derived cervical squamous cell carcinoma organoids and synergistic effect with paclitaxel.Virol J. 2024 Oct 5;21(1):245. doi: 10.1186/s12985-024-02502-y. Virol J. 2024. PMID: 39369233 Free PMC article.
-
Developing Vaccines in Pancreatic Adenocarcinoma: Trials and Tribulations.Curr Oncol. 2024 Aug 23;31(9):4855-4884. doi: 10.3390/curroncol31090361. Curr Oncol. 2024. PMID: 39329989 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. - PubMed
-
- Holsti LR, Mattson K, Niiranen A, et al. Enhancement of radiation effects by alpha interferon in the treatment of small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1987;13:1161‐1166. - PubMed
-
- Ismail A, Van Groeningen CJ, Hardcastle A, et al. Modulation of fluorouracil cytotoxicity by interferon‐alpha and ‐gamma. Mol Pharmacol. 1998;53:252‐261. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

