Standard RUTF vs. locally-made RUSF for acutely malnourished children: A quasi-experimental comparison of the impact on growth and compliance in a rural community of Pakistan
- PMID: 37437065
- PMCID: PMC10337979
- DOI: 10.1371/journal.pone.0287962
Standard RUTF vs. locally-made RUSF for acutely malnourished children: A quasi-experimental comparison of the impact on growth and compliance in a rural community of Pakistan
Abstract
Background: The reduction in severe and moderate acute malnutrition (SAM and MAM) rates in Pakistan has been sub-optimal compared to other low-and middle-income countries (LMICs). Specially-formulated products have been designed globally to manage SAM and MAM, such as ready-to-use therapeutic food (RUTF) and ready-to-use supplementary food (RUSF), with variable efficacies. RUTF is primarily produced and patented in industrialized countries, raising supply challenges in resource-constrained regions with a high burden of acute malnutrition. RUSF minimizes costs by using locally-available ingredients while providing similar nutritional value. In this study, we compared the efficacy, side effects, and compliance of two months of supplementation with either RUTF or RUSF.
Methods: Children aged nine months in the rural district of Matiari, Pakistan, with a weight-for-height z-score (WHZ) <-2 received either RUTF (500 kcal sachet) for two months in 2015 or RUSF (520 kcal sachet) for two months in 2018.
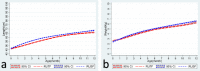
Results: The RUSF group had a higher height gain and mid-upper arm circumferences (MUAC) score. Higher compliance was noted with lower side effects in the RUSF group. A higher compliance rate did correlate with the growth parameters in respective groups.
Conclusion: Our study found that both RUTF and RUSF partially improve the anthropometric status of acutely malnourished children, with neither being superior to the other.
Copyright: © 2023 Sarfraz et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Effectiveness of interventions to manage acute malnutrition in children under 5 years of age in low- and middle-income countries: A systematic review.Campbell Syst Rev. 2020 Apr 9;16(2):e1082. doi: 10.1002/cl2.1082. eCollection 2020 Jun. Campbell Syst Rev. 2020. PMID: 37131422 Free PMC article. Review.
-
Simplifying and optimising the management of uncomplicated acute malnutrition in children aged 6-59 months in the Democratic Republic of the Congo (OptiMA-DRC): a non-inferiority, randomised controlled trial.Lancet Glob Health. 2022 Apr;10(4):e510-e520. doi: 10.1016/S2214-109X(22)00041-9. Lancet Glob Health. 2022. PMID: 35303461 Clinical Trial.
-
Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age.Cochrane Database Syst Rev. 2019 May 15;5(5):CD009000. doi: 10.1002/14651858.CD009000.pub3. Cochrane Database Syst Rev. 2019. PMID: 31090070 Free PMC article.
-
A simplified, combined protocol versus standard treatment for acute malnutrition in children 6-59 months (ComPAS trial): A cluster-randomized controlled non-inferiority trial in Kenya and South Sudan.PLoS Med. 2020 Jul 9;17(7):e1003192. doi: 10.1371/journal.pmed.1003192. eCollection 2020 Jul. PLoS Med. 2020. PMID: 32645109 Free PMC article. Clinical Trial.
-
Ready-to-use therapeutic/supplementary foods from local food resources: Technology accessibility, program effectiveness, and sustainability, a review.Heliyon. 2023 Nov 19;9(12):e22478. doi: 10.1016/j.heliyon.2023.e22478. eCollection 2023 Dec. Heliyon. 2023. PMID: 38046154 Free PMC article. Review.
Cited by
-
A shared group of bacterial taxa in the duodenal microbiota of undernourished Pakistani children with environmental enteric dysfunction.mSphere. 2024 Jun 25;9(6):e0019624. doi: 10.1128/msphere.00196-24. Epub 2024 May 14. mSphere. 2024. PMID: 38742887 Free PMC article.
-
Current evidence on the effectiveness of Ready-to-Use Supplementary Foods in children with moderate acute malnutrition: a systematic review and meta-analysis.J Nutr Sci. 2024 Jan 3;12:e130. doi: 10.1017/jns.2023.114. eCollection 2023. J Nutr Sci. 2024. PMID: 38179261 Free PMC article.
-
Epidemiology and clinical characteristics of acute malnutrition among under-5 children attending a rural hospital in the Democratic Republic of Congo: a cross-sectional study.Ann Med Surg (Lond). 2024 Jun 21;86(8):4402-4409. doi: 10.1097/MS9.0000000000002264. eCollection 2024 Aug. Ann Med Surg (Lond). 2024. PMID: 39118709 Free PMC article.
References
-
- ICF NI of PS (NIPS) [Pakistan] and. Pakistan Demographic and Health Survey (PDHS) 2017–18. Natl Inst Popul Stud Islam Pakistan, United States Agency Int Dev [Internet]. 2018; Available from: https://dhsprogram.com/pubs/pdf/FR354/FR354.pdf
-
- Medoua GN, Ntsama PM, Ndzana ACA, Essa’a VJ, Tsafack JJT, Dimodi HT. Recovery rate of children with moderate acute malnutrition treated with ready-to-use supplementary food (RUSF) or improved corn–soya blend (CSB+): a randomized controlled trial. Public Health Nutr. 2016;19(2):363–70. doi: 10.1017/S1368980015001238 - DOI - PMC - PubMed
-
- Karakochuk C, van den Briel T, Stephens D, Zlotkin S. Treatment of moderate acute malnutrition with ready-to-use supplementary food results in higher overall recovery rates compared with a corn-soya blend in children in southern Ethiopia: an operations research trial. Am J Clin Nutr. 2012;96(4):911–6. doi: 10.3945/ajcn.111.029744 - DOI - PubMed
-
- Greiner T. The advantages, disadvantages and risks of ready-to-use foods. Breastfeed Briefs. 2014;56(57):1–22.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

