Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis
- PMID: 37436070
- PMCID: PMC10337265
- DOI: 10.1002/14651858.CD011535.pub6
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis
Abstract
Background: Psoriasis is an immune-mediated disease with either skin or joints manifestations, or both, and it has a major impact on quality of life. Although there is currently no cure for psoriasis, various treatment strategies allow sustained control of disease signs and symptoms. The relative benefit of these treatments remains unclear due to the limited number of trials comparing them directly head-to-head, which is why we chose to conduct a network meta-analysis.
Objectives: To compare the benefits and harms of non-biological systemic agents, small molecules, and biologics for people with moderate-to-severe psoriasis using a network meta-analysis, and to provide a ranking of these treatments according to their benefits and harms.
Search methods: For this update of the living systematic review, we updated our searches of the following databases monthly to October 2022: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase.
Selection criteria: Randomised controlled trials (RCTs) of systemic treatments in adults over 18 years with moderate-to-severe plaque psoriasis, at any stage of treatment, compared to placebo or another active agent. The primary outcomes were: proportion of participants who achieved clear or almost clear skin, that is, at least Psoriasis Area and Severity Index (PASI) 90; proportion of participants with serious adverse events (SAEs) at induction phase (8 to 24 weeks after randomisation).
Data collection and analysis: We conducted duplicate study selection, data extraction, risk of bias assessment, and analyses. We synthesised data using pairwise and network meta-analysis (NMA) to compare treatments and rank them according to effectiveness (PASI 90 score) and acceptability (inverse of SAEs). We assessed the certainty of NMA evidence for the two primary outcomes and all comparisons using CINeMA, as very low, low, moderate, or high. We contacted study authors when data were unclear or missing. We used the surface under the cumulative ranking curve (SUCRA) to infer treatment hierarchy, from 0% (worst for effectiveness or safety) to 100% (best for effectiveness or safety).
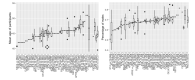
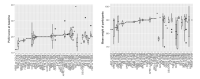
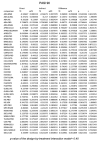
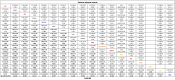
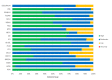
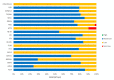
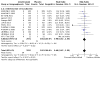
Main results: This update includes an additional 12 studies, taking the total number of included studies to 179, and randomised participants to 62,339, 67.1% men, mainly recruited from hospitals. Average age was 44.6 years, mean PASI score at baseline was 20.4 (range: 9.5 to 39). Most studies were placebo-controlled (56%). We assessed a total of 20 treatments. Most (152) trials were multicentric (two to 231 centres). One-third of the studies (65/179) had high risk of bias, 24 unclear risk, and most (90) low risk. Most studies (138/179) declared funding by a pharmaceutical company, and 24 studies did not report a funding source. Network meta-analysis at class level showed that all interventions (non-biological systemic agents, small molecules, and biological treatments) showed a higher proportion of patients reaching PASI 90 than placebo. Anti-IL17 treatment showed a higher proportion of patients reaching PASI 90 compared to all the interventions. Biologic treatments anti-IL17, anti-IL12/23, anti-IL23, and anti-TNF alpha showed a higher proportion of patients reaching PASI 90 than the non-biological systemic agents. For reaching PASI 90, the most effective drugs when compared to placebo were (SUCRA rank order, all high-certainty evidence): infliximab (risk ratio (RR) 49.16, 95% CI 20.49 to 117.95), bimekizumab (RR 27.86, 95% CI 23.56 to 32.94), ixekizumab (RR 27.35, 95% CI 23.15 to 32.29), risankizumab (RR 26.16, 95% CI 22.03 to 31.07). Clinical effectiveness of these drugs was similar when compared against each other. Bimekizumab and ixekizumab were significantly more likely to reach PASI 90 than secukinumab. Bimekizumab, ixekizumab, and risankizumab were significantly more likely to reach PASI 90 than brodalumab and guselkumab. Infliximab, anti-IL17 drugs (bimekizumab, ixekizumab, secukinumab, and brodalumab), and anti-IL23 drugs except tildrakizumab were significantly more likely to reach PASI 90 than ustekinumab, three anti-TNF alpha agents, and deucravacitinib. Ustekinumab was superior to certolizumab. Adalimumab, tildrakizumab, and ustekinumab were superior to etanercept. No significant difference was shown between apremilast and two non-biological drugs: ciclosporin and methotrexate. We found no significant difference between any of the interventions and the placebo for the risk of SAEs. The risk of SAEs was significantly lower for participants on methotrexate compared with most of the interventions. Nevertheless, the SAE analyses were based on a very low number of events with very low- to moderate-certainty evidence for all the comparisons. The findings therefore have to be viewed with caution. For other efficacy outcomes (PASI 75 and Physician Global Assessment (PGA) 0/1), the results were similar to the results for PASI 90. Information on quality of life was often poorly reported and was absent for several of the interventions.
Authors' conclusions: Our review shows that, compared to placebo, the biologics infliximab, bimekizumab, ixekizumab, and risankizumab were the most effective treatments for achieving PASI 90 in people with moderate-to-severe psoriasis on the basis of high-certainty evidence. This NMA evidence is limited to induction therapy (outcomes measured from 8 to 24 weeks after randomisation), and is not sufficient for evaluating longer-term outcomes in this chronic disease. Moreover, we found low numbers of studies for some of the interventions, and the young age (mean 44.6 years) and high level of disease severity (PASI 20.4 at baseline) may not be typical of patients seen in daily clinical practice. We found no significant difference in the assessed interventions and placebo in terms of SAEs, and the safety evidence for most interventions was very low to moderate quality. More randomised trials directly comparing active agents are needed, and these should include systematic subgroup analyses (sex, age, ethnicity, comorbidities, psoriatic arthritis). To provide long-term information on the safety of treatments included in this review, an evaluation of non-randomised studies is needed. Editorial note: This is a living systematic review. Living systematic reviews offer a new approach to review updating, in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Emilie Sbidian: has declared no conflict of interest.
Anna Chaimani: has declared no conflict of interest.
Robin Guelimi: has declared no conflict of interest.
Ignacio Garcia‐Doval: reports payment from Novartis for a presentation unrelated to psoriasis; personal payment. IG‐D also reports receiving meeting expenses from Janssen for the Spanish Academy of Dermatology Annual Congress, personal payment; and payment from UCB (Union Chimique Belge), personal payment.
Camille Hua: has declared no conflict of interest.
Carolyn Hughes: has declared no conflict of interest.
Luigi Naldi: is a contracting member of the EMA PSOLAR Registry Steering Committee (Janssen) and RePhlect European Study Steering Committee (BMS). He received honorarium for participation in advisory board activities from BMS, Boehringer Ingelheim, Leo pharma.
Maria Kinberger: has declared no conflict of interest.
Sivem Afach: has declared no conflict of interest.
Laurence Le Cleach: has declared no conflict of interest.
Figures
























































































Update of
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2022 May 23;5(5):CD011535. doi: 10.1002/14651858.CD011535.pub5. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2023 Jul 12;7:CD011535. doi: 10.1002/14651858.CD011535.pub6. PMID: 35603936 Free PMC article. Updated. Review.
Comment in
-
Systemic Pharmacologic Treatments for Plaque Psoriasis.Am Fam Physician. 2024 Feb;109(2):116-117. Am Fam Physician. 2024. PMID: 38393794 No abstract available.
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2022 May 23;5(5):CD011535. doi: 10.1002/14651858.CD011535.pub5. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2023 Jul 12;7:CD011535. doi: 10.1002/14651858.CD011535.pub6. PMID: 35603936 Free PMC article. Updated. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated. Review.
-
Systemic treatments for eczema: a network meta-analysis.Cochrane Database Syst Rev. 2020 Sep 14;9(9):CD013206. doi: 10.1002/14651858.CD013206.pub2. Cochrane Database Syst Rev. 2020. PMID: 32927498 Free PMC article.
Cited by
-
Risankizumab: A Review in Moderate to Severe Plaque Psoriasis.Drugs. 2020 Aug;80(12):1235-1245. doi: 10.1007/s40265-020-01357-1. Drugs. 2020. PMID: 32632826 Free PMC article. Review.
-
Short term efficacy of biological treatment for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis.Arch Dermatol Res. 2024 Oct 18;316(10):699. doi: 10.1007/s00403-024-03398-y. Arch Dermatol Res. 2024. PMID: 39424649
-
The Efficacy of Lactocare® Synbiotic on the Clinical Symptoms in Patients with Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial.Dermatol Res Pract. 2022 Oct 7;2022:4549134. doi: 10.1155/2022/4549134. eCollection 2022. Dermatol Res Pract. 2022. PMID: 36249714 Free PMC article.
-
Secukinumab Causing Medication-Related Osteonecrosis of the Jaw, in a Patient Diagnosed with Psoriasis and Rheumatoid Arthritis.Psoriasis (Auckl). 2024 Sep 23;14:115-120. doi: 10.2147/PTT.S490982. eCollection 2024. Psoriasis (Auckl). 2024. PMID: 39347517 Free PMC article.
-
Home- vs Office-Based Narrowband UV-B Phototherapy for Patients With Psoriasis: The LITE Randomized Clinical Trial.JAMA Dermatol. 2024 Dec 1;160(12):1320-1328. doi: 10.1001/jamadermatol.2024.3897. JAMA Dermatol. 2024. PMID: 39319513 Clinical Trial.
References
References to studies included in this review
ACCEPT 2010 {published data only}
-
- Griffiths CE, Strober BE, Van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. New England Journal of Medicine 2010;362(2):118-28. [CENTRAL: CN-00734856] [PMID: ] - PubMed
-
- Young MS, Horn EJ, Cather JC. The ACCEPT study: ustekinumab versus etanercept in moderate-to-severe psoriasis patients. Expert Review of Clinical Immunology 2011;7(1):9-13. [CENTRAL: CN-00780322] [PMID: ] - PubMed
ADACCESS 2018 {published data only}
-
- Blauvelt A, Lacour J, Fowler JF, Schuck E, Jauch-Lembach J, Balfour A, et al. Long-term efficacy, safety, and immunogenicity data from a phase III confirmatory study comparing GP2017, a proposed biosimilar, with reference adalimumab. United European Gastroenterology Journal 2017;5(Suppl 1):A301. [CENTRAL: CN-01439136]
-
- Blauvelt A, Lacour JP, Fowler JF Jr, Weinberg JM, Gospodinov D, Schuck E, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. British Journal of Dermatology 2018;179(3):623-31. [CENTRAL: CN-01617973] - PubMed
-
- NCT02016105. Study to demonstrate equivalent efficacy and to compare safety of biosimilar adalimumab (GP2017) and Humira (ADACCESS). clinicaltrials.gov/show/nct02016105 (first received 19 December 2013). [CENTRAL: CN-01479872]
AFFIRM 2022 {published data only}
-
- Mrowietz U, Kircik L, Reich K, Munjal S, Shenoy S, Lebwohl M, et al. Tepilamide fumarate (PPC-06) extended release tablets in patients with moderate-to-severe plaque psoriasis: safety and efficacy results from the randomized, double-blind, placebo-controlled AFFIRM Study. Journal of Clinical and Aesthetic Dermatology 2022;15(1):53-8. [PMID: ] - PMC - PubMed
-
- NCT03421197. A study to assess the efficacy and safety of PPC-06 (tepilamide fumarate). clinicaltrials.gov/show/nct03421197 (first received 5 February 2018).
Akcali 2014 {published data only}
-
- Akcali C, Guven EH, Kirtak N, Inaloz HS, Ozgoztasi O, Guvenc U. Serum concentrations of interleukin-2 and tumour necrosis factor-alpha under cyclosporine versus acitretin treatment in plaque-type psoriasis. Journal of International Medical Research 2014;42(5):1118-22. [CENTRAL: CN-01114333] [PMID: ] - PubMed
Al‐Hamamy 2014 {published data only}
-
- Al-Hamamy HR, Al-Mashhadani SA, Mustafa IN. Comparative study of the effect of narrowband ultraviolet B phototherapy plus methotrexate vs. narrowband ultraviolet B alone and methotrexate alone in the treatment of plaque-type psoriasis. International Journal of Dermatology 2014;53(12):1531-5. [CENTRAL: CN-01089920] [PMID: ] - PubMed
ALLURE 2021 {unpublished data only}
-
- EUCTR2015-005170-38-BE. Study of efficacy and safety of secukinumab 2 mL pre-filled syringe (300 mg) in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-005170-38-BE (first received 29 July 2016).
-
- NCT02748863. Study of secukinumab with 2 mL pre-filled syringes (ALLURE). clinicaltrials.gov/ct2/show/NCT02748863 (first received 22 April 2016).
-
- Sigurgeirsson B, Schakel K, Hong CH, Effendy I, Placek W, Rich P, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. Journal of Dermatological Treatment 2022;33(3):1718‐26. - PubMed
-
- Sigurgeirsson B, Schäkel K, Hong CH, Effendy I, Placek W, Rich P, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. Journal of Dermatological Treatment 2021 Apr 26 [Epub ahead of print]. [DOI: 10.1080/09546634.2021.1902925] - DOI - PubMed
AlMutairi 2021 {published data only}
-
- AlMutairi N, Eassa BI. A randomized controlled ixekizumab vs secukinumab trial to study the impact on sexual activity in adult patients with genital psoriasis. Expert Opinion on Biological Therapy 2021;21(2):297-8. - PubMed
AMAGINE‐1 2016 {published data only}
-
- Armstrong A, Strober B, Ehst BD, Jacobson A. 15921 Absolute PASI response up to 52 weeks with brodalumab in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB164.
-
- Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized,double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2016;175(2):273-86. [CENTRAL: CN-01208651] - PubMed
AMAGINE‐2 2015 {published data only}
-
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New England Journal of Medicine 2015;373(14):1318-28. [CENTRAL: CN-01089800] [PMID: ] - PubMed
-
- Menter A, Sobell J, Silverberg JI, Lebwohl M, Rastogi S, Pillai R, et al. Long-term efficacy of brodalumab for the treatment of moderate-to-severe psoriasis: data from a pivotal Phase III clinical trial. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S26-7. [CENTRAL: CN-01713709]
-
- Papp KA, Lebwohl MG, Green LJ, Yamauchi PS, Rastogi S, Israel R, et al. Maintenance of clinical efficacy in moderate-to-severe plaque psoriasis: a 52-week evaluation of brodalumab in three multicenter, double-blind studies of 4363 subjects. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S23-4. [CENTRAL: CN-01713074]
AMAGINE‐3 2015 {published data only}
-
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New England Journal of Medicine 2015;373(14):1318-28. [CENTRAL: CN-01089800] [PMID: ] - PubMed
Asahina 2010 {published data only}
-
- Asahina A, Nakagawa H, Etoh T, Ohtsuki M, Adalimumab M04-688 Study Group. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. Journal of Dermatology 2010;37(4):299-310. [CENTRAL: CN-00762123] [PMID: ] - PubMed
Asawanonda 2006 {published data only}
-
- Asawanonda P, Nateetongrungsak Y. Methotrexate plus narrowband UVB phototherapy versus narrowband UVB phototherapy alone in the treatment of plaque-type psoriasis: a randomized, placebo-controlled study. Journal of the American Academy of Dermatology 2006;54(6):1013-8. [CENTRAL: CN-00556468] [PMID: ] - PubMed
Augustin 2022 {published data only}
-
- Augustin M, Reich K, Yamauchi P, Pinter A, Bagel J, Dahale S, et al. Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial. British Journal of Dermatology 2022;186(6):942-54. [PMID: ] - PubMed
-
- NCT03504852. Efficacy and safety of 2 secukinumab regimens in 90 kg or higher subjects with moderate to severe chronic plaque-type psoriasis. clinicaltrials.gov/show/nct03504852 (first received 20 April 2018).
AURIEL‐PsO 2020 {published data only}
-
- Hercogova J, Papp KA, Chyrok V, Ullmann M, Vlachos P, Edwards CJ. AURIEL-PsO: a randomised, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. British Journal of Dermatology 2020;182(2):316-26. - PMC - PubMed
-
- NCT02660580. MSB11022 in moderate to severe chronic plaque psoriasis (AURIEL-PsO). clinicaltrials.gov/show/nct02660580 (first received 21 January 2016). [CENTRAL: CN-01555234]
Bachelez 2015 {published data only}
-
- Bachelez H, Van de Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386(9993):552-61. [CENTRAL: CN-01091031] [PMID: ] - PubMed
-
- Valenzuela F, Paul C, Mallbris L, Tan H, Papacharalambous J, Valdez H, et al. Tofacitinib versus etanercept or placebo in patients with moderate to severe chronic plaque psoriasis: patient-reported outcomes from a phase 3 study. Journal of the European Academy of Dermatology and Venereology 2016;30(10):1753-9. [CENTRAL: CN-01368560] [PMID: ] - PMC - PubMed
Bagel 2012 {published data only}
-
- Bagel J, Kricorian G, Klekotka P, Tyring S. Etanercept therapy for moderate to severe plaque psoriasis with involvement of the scalp. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN-00843659] - PubMed
-
- Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. Journal of the American Academy of Dermatology 2012;67(1):86-92. [CENTRAL: CN-00870940] [PMID: ] - PubMed
Barker 2011 {published data only}
-
- Barker J, Hoffmann M, Wozel G, Ortonne JP, Zheng H, Van Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). British Journal of Dermatology 2011;165(5):1109-17. [CENTRAL: CN-00805921] [PMID: ] - PubMed
BE ABLE 1 2018 {published data only}
-
- Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, et al. Bimekizumab for patients with moderate-to-severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled phase 2b extension study. Journal of the American Academy of Dermatology 2020;83(5):1367-74. [DOI: 10.1016/j.jaad.2020.05.105] [PMID: ] - DOI - PubMed
-
- NCT02905006. Study to evaluate safety and efficacy of different doses of bimekizumab in patients with chronic plaque psoriasis (BE ABLE 1). clinicaltrials.gov/show/nct02905006 (first received 19 September 2016). [CENTRAL: CN-01520880]
-
- Papp KA, Merola JF, Gottlieb AB, Griffiths CE, Cross N, Peterson L, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. Journal of the American Academy of Dermatology 2018;79(2):277-86.e10. [CENTRAL: CN-01665198] - PubMed
BE RADIANT 2021 {published data only}
-
- Augustin M, Gooderham M, Gottlieb AB. 31069 Bimekizumab versus secukinumab in plaque psoriasis: reduction in body surface area affected by psoriasis translates into benefits in patient-perceived itching, skin pain, and scaling and health-related quality of life in the BE RADIANT phase 3b trial. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB25.
-
- Augustin M, Gottlieb AB, Laws P, Wu JJ, Sebastian M, Eusebi P, et al. Bimekizumab versus secukinumab in plaque psoriasis: higher efficacy translates into benefits in patient-perceived symptoms and health-related quality of life in the BE RADIANT multicenter, randomized, double blinded phase 3b trial. Journal of Clinical and Aesthetic Dermatology 2022;15(4 Suppl 1):S23-4.
-
- NCT03536884. A study to evaluate the efficacy and safety of bimekizumab compared to an active comparator in adult subjects with moderate to severe chronic plaque psoriasis (BE RADIANT). clinicaltrials.gov/show/nct03536884 (first received 25 May 2018).
-
- Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus secukinumab in plaque psoriasis. New England Journal of Medicine 2021;385(2):142-52. - PubMed
BE READY 2021 {published data only}
-
- Blauvelt A, Wu JJ, Reich K, Gooderham M, Lebwohl M, White K, et al. 27380 Bimekizumab efficacy in patients with moderate to severe plaque psoriasis during the randomized withdrawal and retreatment phase of BE READY, a phase 3 trial. Journal of the American Academy of Dermatology 2021;85(3 Suppl):AB139.
-
- Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021;397(10273):475-86 Erratum in: Lancet 2021; 397(10288); 1182. [PMID: ] - PubMed
-
- NCT03410992. A study with an initial treatment period followed by a randomized-withdrawal period to evaluate the efficacy and safety of bimekizumab in adult subjects with moderate to severe chronic plaque psoriasis (BE READY). clinicaltrials.gov/show/nct03410992 (first received 25 January 2018).
BE SURE 2021 {published data only}
-
- NCT03412747. A study to evaluate the efficacy and safety of bimekizumab in adult subjects with moderate to severe chronic plaque psoriasis (BE SURE). clinicaltrials.gov/show/nct03412747 (first received 26 January 2018).
-
- Warren RB, Blauvelt A, Bagel J, Papp K, Yamauchi P, Armstrong A, et al. Bimekizumab efficacy and safety versus adalimumab in patients with moderate-to-severe plaque psoriasis: results from a multicenter, randomized, double-blinded active comparator-controlled phase III trial (BE SURE). Journal of Clinical and Aesthetic Dermatology 2021;14(5 Suppl 1):S21-2.
-
- Warren RB, Blauvelt A, Bagel J, Papp KA, Yamauchi P, Armstrong A, et al. Bimekizumab versus adalimumab in plaque psoriasis. New England Journal of Medicine 2021;385(2):130-41. - PubMed
BE VIVID 2021 {published data only}
-
- Gordon K, Foley P, Rich P, Duffin K, Pinter A, Griffiths CEM, et al. Bimekizumab versus ustekinumab in plaque psoriasis: lasting efficacy translates to rapid and sustained improvements in quality of life in the BE VIVID multicentre, randomized, double-blinded phase III trial. British Journal of Dermatology 2022;187(1):e38-9.
-
- Gordon K, Foley P, Rich P, Duffn K, Pinter A, Griffths CEM, et al. Bimekizumab versus ustekinumab in plaque psoriasis: lasting efficacy translates to rapid and sustained improvements in quality of life in the BE VIVID multicenter, randomized, double-blinded Phase III trial. Journal of Clinical and Aesthetic Dermatology 2021;14(5 Suppl 1):S23-4.
-
- NCT03370133. A study to evaluate the efficacy and safety of bimekizumab compared to placebo and an active comparator in adult subjects with moderate to severe chronic plaque psoriasis (BE VIVID). clinicaltrials.gov/show/nct03370133 (first received 12 December 2017).
-
- Papp K, Lebwohl M, Gottlieb AB, Sebastian M, Langley R, Okubo Y, et al. Bimekizumab for the treatment of moderate-to-severe plaque psoriasis with scalp, nail and palmoplantar involvement through 52 weeks: post-hoc analysis from the BE VIVID Phase III trial. Journal of Clinical and Aesthetic Dermatology 2021;14(5 Suppl 1):S22.
-
- Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021;397(10273):487-98. - PubMed
Bissonnette 2013 {published data only}
-
- Bissonnette R, Tardif J-C, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circulation: Cardiovascular Imaging 2013;6(1):83-90. [CENTRAL: CN-00906599] [EMBASE: 2013325307] - PubMed
Blauvelt 2021a {published data only}
-
- Blauvelt A, Gordon KB, Lee P, Bagel J, Sofen H, Lockshin B, et al. Efficacy, safety, usability, and acceptability of risankizumab 150 mg formulation administered by prefilled syringe or by an autoinjector for moderate to severe plaque psoriasis. Journal of Dermatological Treatment 2022;33(4):2085-93. - PubMed
-
- NCT03875482. A study to assess safety and efficacy of risankizumab using a new formulation in participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03875482 (first received 14 March 2019).
BRIDGE 2017 {published data only}
-
- Mrowietz U, Szepietowski JC, Loewe R, Van de Kerkhof P, Lamarca R, Ocker WG, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm® - and placebo-controlled trial (BRIDGE). British Journal of Dermatology 2017;176(3):615-23 Corrigendum to: British Journal of Dermatology 2018; 178(1); 308. [CENTRAL: CN-01336917] [PMID: ] - PubMed
-
- Van de Kerkhof PCM, Loewe R, Mrowietz U, Falques M, Pau-Charles I, Szepietowski JC. Quality of life outcomes in adults with moderate-to-severe plaque psoriasis treated with dimethylfumarate (DMF): a post hoc analysis of the BRIDGE study. Journal of the European Academy of Dermatology & Venereology 2020;34(1):119-26. - PMC - PubMed
-
- Van de Kerkhof PCM, Loewe R, Mrowietz U, Falques M, Pau-Charles I, Szepietowski JC. Quality of life outcomes in adults with moderate-to-severe plaque psoriasis treated with dimethylfumarate (DMF): a post-hoc analysis of the BRIDGE study. Journal of the European Academy of Dermatology & Venereology 2020;34(1):119-26. - PMC - PubMed
Cai 2016 {published data only}
-
- Cai L, Gu J, Zheng J, Zheng M, Wang G, Xi LY, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. Journal of the European Academy of Dermatology and Venereology 2016;31(1):89-95. [CENTRAL: CN-01248561] [PMID: ] - PMC - PubMed
Cai 2020 {published data only}
-
- Anonymous. Secukinumab 300 mg showed faster and higher efficacy in Chinese moderate to severe plaque psoriasis patients. Journal of the American Academy of Dermatology 2019;81(4 Suppl 1):AB445.
-
- NCT03066609. Study of efficacy and safety of secukinumab in subjects with moderate to severe chronic plaque-type psoriasis. clinicaltrials.gov/show/nct03066609 (first received 28 February 2017).
-
- TCTR20161028001. A randomised, double-blind, placebo controlled, multicenter study of subcutaneous secukinumab, to demonstrate efficacy after twelve weeks of treatment and to assess safety, tolerability and long-term efficacy up to one year in subjects with moderate to severe chronic plaque-type psoriasis with or without psoriatic arthritis comorbidity. www.thaiclinicaltrials.org/show/TCTR20161028001 (first received 28 February 2017).
Cai 2022 {published data only}
CALYPSO 2018 {published data only}
-
- CTRI/2018/03/012598. Comparative double-blind study of the efficacy and safety of BCD-057 and Humira® in patients with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/03/012598 (first received 15 March 2018).
-
- Korotaeva TV, Samtsov AV, Bakulev AL, Kokhan MM, Minullin IK, Vylegzhanina OA, et al. Comparative efficacy and safety of adalimumab biosimilar (BCD-057) and innovator in patients with psoriasis vulgaris. Results of the BCD-057-2/CALYPSO phase III international, multicenter, randomized double-blind clinical trial [Сравнительная эффективностьи безопасность биоаналога адалимумаба (BCD-057) и оригинального адалимумаба у пациентов с вульгарным псориазом. Результаты BCD-057-2/CALYPSO – международного многоцентрового рандомизированного двойного слепого клинического исследования III фазы]. Modern Rheumatology Journal 2018;12(4):71-84. [DOI: 10.14412/1996-7012-2018-4-71-84] - DOI
-
- NCT02762955. Comparative clinical trial of efficacy and safety of BCD-057 and Humira® in patients with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02762955 (first received 5 May 2016).
Caproni 2009 {published data only}
-
- Caproni M, Antiga E, Melani L, Volpi W, Bianco E, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. Journal of Clinical Immunology 2009;29(2):210-4. [CENTRAL: CN-00685566] [PMID: ] - PubMed
CARIMA 2019 {unpublished data only}
-
- NCT02559622. Evaluation of cardiovascular risk markers in psoriasis patients treated with secukinumab (CARIMA). clinicaltrials.gov/ct2/show/NCT02559622 (first received 4 August 2015).
-
- Von Stebut E, Reich K, Thaçi D, Koenig W, Pinter A, Korber A, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. Journal of Investigative Dermatology 2019;139(5):1054-62. - PubMed
Cestari 2021 {published data only}
-
- Cestari TF, DaSilva Souza C, Azulay-Abulafia L, Romiti R, Carvalho AVE, Silva de Castro CC, et al. 26197 Efficacy and safety of risankizumab vs methotrexate in patients with moderate-to-severe plaque psoriasis: results from the 28-week randomized, double-blind period of an ongoing phase 3 study in Brazil. Journal of the American Academy of Dermatology 2021;85(3 Suppl):AB88.
CHAMPION 2008 {published data only}
-
- Navarini AA, Poulin Y, Menter A, Gu Y, Teixeira HD. Analysis of body regions and components of PASI scores during adalimumab or methotrexate treatment for patients with moderate-to-severe psoriasis. Journal of Drugs in Dermatology 2014;13(5):554-62. [CENTRAL: CN-00993155] [PMID: ] - PubMed
-
- Prussick R, Unnebrink K, Valdecantos WC. Efficacy of adalimumab compared with methotrexate or placebo stratified by baseline BMI in a randomized placebo-controlled trial in patients with psoriasis. Journal of Drugs in Dermatology 2015;14(8):864-8. [CENTRAL: CN-01132989] [PMID: ] - PubMed
-
- Reich K, Signorovitch J, Ramakrishnan K, Yu AP, Wu EQ, Gupta SR, et al. Benefit-risk analysis of adalimumab versus methotrexate and placebo in the treatment of moderate to severe psoriasis: comparison of adverse event-free response days in the CHAMPION trial. Journal of the American Academy of Dermatology 2010;63(6):1011-8. [CENTRAL: CN-00791143] [PMID: ] - PubMed
-
- Revicki D, Willian MK, Saurat JH, Papp KA, Ortonne JP, Sexton C, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. British Journal of Dermatology 2008;158(3):549-57. [CENTRAL: CN-00628564] [PMID: ] - PubMed
-
- Saurat JH, Langley RG, Reich K, Unnebrink K, Sasso EH, Kampman W. Relationship between methotrexate dosing and clinical response in patients with moderate to severe psoriasis: subanalysis of the CHAMPION study. British Journal of Dermatology 2011;165(2):399-406. [CENTRAL: CN-00800067] [PMID: ] - PubMed
CHANGE 2021 {published data only}
-
- NCT03331835. A trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03331835 (first received 6 November 2017).
-
- Pinter A, Hoffmann M, Reich K, Augustin M, Kaplan K, Gudjonsdottir SD, et al. A phase 4, randomised, head-to-head trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate-to-severe plaque psoriasis (CHANGE). Journal of the European Academy of Dermatology & Venereology 2020;16:16. - PubMed
-
- Pinter A, Hoffmann M, Reich K, Augustin M, Kaplan K, Gudjonsdottir SD, et al. A phase 4, randomized, head-to-head trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate-to-severe plaque psoriasis (CHANGE). Journal of the European Academy of Dermatology and Venereology 2021;35(3):701-11. - PubMed
Chaudhari 2001 {published data only}
-
- Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001;357(9271):1842-7. [CENTRAL: CN-00348743] [PMID: ] - PubMed
-
- Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque-type psoriasis. Journal of the American Academy of Dermatology 2003;48(6):829-35. [CENTRAL: CN-00438058] [PMID: ] - PubMed
-
- Gottlieb AB, Masud S, Ramamurthi R, Abdulghani A, Romano P, Chaudhari U, et al. Pharmacodynamic and pharmacokinetic response to anti-tumor necrosis factor-alpha monoclonal antibody (infliximab) treatment of moderate to severe psoriasis vulgaris. Journal of the American Academy of Dermatology 2003;48(1):68-75. [CENTRAL: CN-00466030] [PMID: ] - PubMed
Chladek 2005 {published data only}
-
- Chladek J, Grim J, Martinkova J, Simkova M, Vaneckova J. Low-dose methotrexate pharmacokinetics and pharmacodynamics in the therapy of severe psoriasis. Basic & Clinical Pharmacology & Toxicology 2005;96(3):247-8. [CENTRAL: CN-00513064] [PMID: ] - PubMed
CIMPACT 2018 {unpublished data only}
-
- Lebwohl M, Blauvelt A, Paul C, Sofen H, Weglowska J, Piguet V, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). Journal of the American Academy of Dermatology 2018;79(2):266-76.e5. [CENTRAL: CN-01665155] - PubMed
-
- NCT02346240. Efficacy and safety study of certolizumab pegol (CZP) versus active comparator and placebo in subjects with plaque psoriasis (PSO) (CIMPACT). clinicaltrials.gov/ct2/show/NCT02346240 (first received 20 January 2015). [CENTRAL: CN-01551710]
CIMPASI‐1 2018 {published data only}
-
- Gottlieb AB, Blauvelt A, Thaçi D, Leonardi CL, Poulin Y, Drew J, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). Journal of the American Academy of Dermatology 2018;79(2):302-14.e6. [CENTRAL: CN-01665156] - PubMed
-
- NCT02326298. An efficacy and safety study of two dose levels of certolizumab pegol (CZP) in subjects with plaque psoriasis (PSO). clinicaltrials.gov/show/nct02326298 (first received 29 December 2014). [CENTRAL: CN-01575600]
CIMPASI‐2 2018 {published and unpublished data}
-
- Gottlieb AB, Blauvelt A, Thaçi D, Leonardi CL, Poulin Y, Drew J, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). Journal of the American Academy of Dermatology 2018;79(2):302-14.e6. [CENTRAL: CN-01665156] - PubMed
-
- NCT02326272. A study to evaluate the efficacy and safety of two dose levels of certolizumab pegol (CZP) in subjects with plaque psoriasis (PSO) (CIMPASI-2). clinicaltrials.gov/ct2/show/NCT02326272 (first received 22 December 2014). [CENTRAL: CN-01575599]
CLARITY 2018 {unpublished data only}
-
- Anonymous. Secukinumab is superior to ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: CLARITY, a randomized, controlled, phase 3b trial. Journal of the American Academy of Dermatology 2019;81(4 Supp 1):AB274.
-
- Bagel J, Blauvelt A, Nia J, Hashim P, Patekar M, De Vera A, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). Journal of the European Academy of Dermatology and Venereology 2020;35(1):135-42. - PMC - PubMed
-
- Bagel J, Blauvelt A, Nia J, Hashim P, Patekar M, Vera A, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). Journal of the European Academy of Dermatology & Venereology 2020 May 4 [Epub ahead of print]. [DOI: 10.1111/jdv.16558] - DOI - PMC - PubMed
-
- Bagel J, Nia J, Hashim P, Patekar M, De Vera A, Hugot S, et al. Secukinumab is superior to ustekinumab in clearing skin of patients with moderate-to-severe plaque psoriasis: CLARITY, a randomized, controlled, Phase IIIb trial. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S27-8. [CENTRAL: CN-01713715] - PMC - PubMed
CLEAR 2015 {published data only}
-
- Blauvelt A, Korman N, Mollon P, Zhao Y, Milutinovic M, You R, et al. Secukinumab treatment provides faster and more effective relief from patient-reported quality of life impact than ustekinumab in subjects with moderate to severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S15-6. [CENTRAL: CN-01713052]
-
- Blauvelt A, Reich K, Mehlis S, Vanaclocha F, Sofen H, Abramovits W, et al. Secukinumab demonstrates greater sustained improvements in daily activities and personal relationships than ustekinumab in patients with moderate-to-severe plaque psoriasis: 52-week results from the CLEAR study. Journal of the European Academy of Dermatology and Venereology 2017;31(10):1693-9. [CENTRAL: CN-01615823] - PMC - PubMed
-
- Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. Journal of the American Academy of Dermatology 2017;76(1):60-9.e9. [CENTRAL: CN-01368612] - PubMed
-
- Herranz Pinto P, Rivera R, Blauvelt A, Thaçi D, Oliver Vigueras J. Secukinumab 300mg is more efficacious than ustekinumab 90mg: analysis of the CLEAR study. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S18-9. [CENTRAL: CN-01713059]
-
- Spelman L, Pinto PH, Rivera R, Blauvelt A, Thaçi D, Vigueras JO. Secukinumab 300 mgs is more efficacious than ustekinumab 90 mgs: analysis of patients with body weights over 100kg from the CLEAR study. Australasian Journal of Dermatology 2017;58(Suppl 1):86-7. [CENTRAL: CN-01378828]
Dogra 2012 {published data only}
-
- Dogra S, Krishna V, Kanwar AJ. Efficacy and safety of systemic methotrexate in two fixed doses of 10 mg or 25 mg orally once weekly in adult patients with severe plaque-type psoriasis: a prospective, randomized, double-blind, dose-ranging study. Clinical and Experimental Dermatology 2012;37(7):729-34. [CENTRAL: CN-00879485] [PMID: ] - PubMed
Dogra 2013 {published data only}
-
- Dogra S, Jain A, Kanwar AJ. Efficacy and safety of acitretin in three fixed doses of 25, 35 and 50 mg in adult patients with severe plaque type psoriasis: a randomized, double blind, parallel group, dose ranging study. Journal of the European Academy of Dermatology and Venereology 2013;27(3):e305-11. [CENTRAL: CN-00911790] [EMBASE: 2013118368] - PubMed
Dubertret 1989 {published data only}
-
- Dubertret L, Perussel M, Robiola O, Feutren G. Cyclosporin in psoriasis. A long-term randomized study on 37 patients. Acta Dermato-Venereologica 1989;69(146 Suppl):136. [CENTRAL: CN-00064909] [PMID: ] - PubMed
ECLIPSE 2019 {published data only}
-
- Armstrong A, Blauvelt A, Flavin S, Hsu MC, Randazzo B, Reich K, et al. Guselkumab demonstrates greater efficacy compared to secukinumab across body weight quartiles and body mass index categories: week 48 results from the ECLIPSE trial. Journal of Clinical and Aesthetic Dermatology 2020;12(5 Suppl):S24.
-
- Blauvelt A, Armstrong A W, Langley R G, Gebauer K, Thaci D, Bagel J, et al. Efficacy of guselkumab versus secukinumab in subpopulations of patients with moderate-to-severe plaque psoriasis: results from the ECLIPSE study. Journal of Dermatological Treatment 2022;33(4):2317-24. - PubMed
-
- Langley RG, Ferris L, Gebauer K, Arenberger P, Gooderham M, Guenther L, et al. Consistent responses to guselkumab by disease region at week 48 in the treatment of moderate-to-severe psoriasis: ECLIPSE trial results. Australasian Journal of Dermatology 2021;62 Suppl 1:58.
-
- Langley RG, Probity KG, Arenberger P, Flavin S, Hsu MC, Li S, et al. 15302 Psoriasis area and severity index component improvements at week 48 in patients treated with guselkumab compared with secukinumab: findings from the ECLIPSE study. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB34.
-
- NCT03090100. A study to evaluate the comparative efficacy of CNTO 1959 (guselkumab) and secukinumab for the treatment of moderate to severe plaque-type psoriasis (ECLIPSE). clinicaltrials.gov/show/nct03090100 (first received 24 March 2017).
EGALITY 2017 {published data only}
-
- Gerdes S, Thaçi D, Griffiths CE, Arenberger P, Poetzl J, Wuerth G, et al. Multiple switches between GP2015, an etanercept biosimilar, with originator product do not impact efficacy, safety and immunogenicity in patients with chronic plaque-type psoriasis: 30-week results from the phase 3, confirmatory EGALITY study. Journal of the European Academy of Dermatology and Venereology 2018;32(3):420-7. [CENTRAL: CN-01643364] - PMC - PubMed
-
- Griffiths CE, Reich K, Thaçi D, Gerdes S, Arenberger P, Kingo K, et al. Switching treatments of etanercept biosimilar GP2015 with originator product does not impact efficacy, safety and immunogenicity in patients with chronic plaque-type psoriasis. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S193. [CENTRAL: CN-01416626]
-
- Griffiths CE, Thaçi D, Gerdes S, Arenberger P, Pulka G, Kingo K, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. British Journal of Dermatology 2017;176(4):928-38. [CENTRAL: CN-01424735] - PubMed
-
- NCT01891864. Study to demonstrate equivalent efficacy and to compare safety of biosimilar etanercept (GP2015) and Enbrel (EGALITY). clinicaltrials.gov/show/nct01891864 (first received 3 July 2013). [CENTRAL: CN-01597476]
Elewski 2016 {published data only}
-
- Elewski BE, Okun MM, Papp K, Baker CS, Crowley JJ, Guillet G, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. Journal of the American Academy of Dermatology 2018;78(1):90-99.e1. [CENTRAL: CN-01443156] [DOI: 10.1016/j.jaad.2017.08.029] - DOI - PubMed
-
- Elewski BE, Rich PA, Behrens F, Guillet G, Geng Z, Reyes Servin O. Primary efficacy and safety of adalimumab in nail psoriasis from the first 26 weeks of a phase-3, randomized, placebo-controlled trial with subanalysis in patients with and without psoriatic arthritis. Annals of the Rheumatic Diseases 2017;76(Suppl 2):1319-20. [CENTRAL: CN-01467694] [DOI: 10.1136/annrheumdis-2017-eular.2148] - DOI
-
- Elewski BE, Rich PA, Behrens F, Guillet G, Geng Z, Servin OR. Primary efficacy and safety of adalimumab in nail psoriasis from the first 26 weeks of a phase-3, randomized, placebo-controlled trial with subanalysis in patients with and without psoriatic arthritis. Acta Dermato-Venereologica 2018;98(Suppl 219):26-7. [CENTRAL: CN-01620195]
-
- Elewski BE, Rich PA, Okun MM, Papp K, Baker CS, Crowley JJ, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase-3, randomized, placebo-controlled trial. Journal of the European Academy of Dermatology and Venereology 2016;30(Suppl 6):65. [CENTRAL: CN-01786943] [EMBASE: 611235503]
Ellis 1991 {published data only}
-
- Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. New England Journal of Medicine 1991;324(5):277-84. [CENTRAL: CN-00072304] [PMID: ] - PubMed
Engst 1994 {published data only}
-
- Engst RH, Bubl R, Huber J, Schober C, Jessberger B. Long-term cyclosporin A for psoriasis. Acta Dermatovenerologica Alpina, Panonica et Adriatica 1994;3(4):188-92. [CENTRAL: CN-00178929] [EMBASE: 1995061459]
ERASURE 2014 {published data only}
-
- Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. Journal of Drugs in Dermatology 2015;14(8):821-33. [CENTRAL: CN-01132993] [PMID: ] - PubMed
-
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis - results of two phase 3 trials. New England Journal of Medicine 2014;371(4):326-38. [CENTRAL: CN-00999505] [PMID: ] - PubMed
-
- Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. Journal of Dermatology 2014;41(12):1039-46. [CENTRAL: CN-01037251] [PMID: ] - PubMed
ESTEEM‐1 2015 {published data only}
-
- Bissonnette R, Pariser DM, Wasel NR, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: results of a pooled analysis from phase II PSOR-005 and phase III Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2016;75(1):99-105. [CENTRAL: CN-01470982] [PMID: ] - PubMed
-
- Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). Journal of the American Academy of Dermatology 2015;73(1):37-49. [CENTRAL: CN-01085116] [PMID: ] - PubMed
-
- Rich P, Gooderham M, Bachelez H, Goncalves J, Day RM, Chen R, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). Journal of the American Academy of Dermatology 2016;74(1):134-42. [CENTRAL: CN-01127546] [PMID: ] - PubMed
ESTEEM‐2 2015 {published data only}
-
- Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). British Journal of Dermatology 2015;173(6):1387-99. [CENTRAL: CN-01133855] [PMID: ] - PubMed
EXPRESS 2005 {published data only}
-
- Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005;366(9494):1367-74. [CENTRAL: CN-00531178] [PMID: ] - PubMed
-
- Reich K, Nestle FO, Papp K, Ortonne JP, Wu Y, Bala M, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. British Journal of Dermatology 2006;154(6):1161-8. [CENTRAL: CN-00565410] [PMID: ] - PubMed
EXPRESS‐II 2007 {published data only}
-
- Feldman SR, Gottlieb AB, Bala M, Wu Y, Eisenberg D, Guzzo C, et al. Infliximab improves health-related quality of life in the presence of comorbidities among patients with moderate-to-severe psoriasis. British Journal of Dermatology 2008;159(3):704-10. [CENTRAL: CN-00668311] [PMID: ] - PubMed
-
- Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2007;56(1):31.e1-15. [CENTRAL: CN-00576883] [PMID: ] - PubMed
-
- Reich K, Nestle FO, Wu Y, Bala M, Eisenberg D, Guzzo C, et al. Infliximab treatment improves productivity among patients with moderate-to-severe psoriasis. European Journal of Dermatology 2007;17(5):381-6. [CENTRAL: CN-00699412] - PubMed
-
- Reich K, Ortonne JP, Kerkmann U, Wang Y, Saurat JH, Papp K, et al. Skin and nail responses after 1 year of infliximab therapy in patients with moderate-to-severe psoriasis: a retrospective analysis of the EXPRESS trial. Dermatology 2010;221(2):172-8. [CENTRAL: CN-01628937] [PMID: ] - PubMed
Fallah Arani 2011 {published data only}
-
- Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. British Journal of Dermatology 2011;164(4):855-61. [CENTRAL: CN-00785701] [PMID: ] - PubMed
-
- ISRCTN76608307. A comparison of the efficacy of oral fumarate and methotrexate therapy in the treatment of severe psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN76608307 (first received 1 September 2006).
FEATURE 2015 {published data only}
-
- Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). British Journal of Dermatology 2015;172(2):484-93. [CENTRAL: CN-01052626] [PMID: ] - PubMed
Feldman 2021 {published data only}
-
- EUCTR2017-003367-35-PL. A multicenter, double-blind, randomized, parallel-group, active control study to compare the efficacy, safety, and immunogenicity of AVT02 versus Humira® in patients with moderate-to-severe chronic plaque psoriasis (ALVOPAD PS). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-003367-35-PL (first received 27 November 2018).
-
- Feldman SR, Reznichenko N, Pulka G, Kingo K, Galdava G, Berti F, et al. Efficacy, safety and immunogenicity of AVT02 versus originator adalimumab in subjects with moderate to severe chronic plaque psoriasis: a multicentre, double-blind, randomised, parallel group, active control, phase III study. BioDrugs 2021;35(6):735-48. [DOI: 10.1007/s40259-021-00502-w] [PMID: ] - DOI - PMC - PubMed
FIXTURE 2014 {published data only}
-
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis - results of two phase 3 trials. New England Journal of Medicine 2014;371(4):326-38. [CENTRAL: CN-00999505] [PMID: ] - PubMed
Flytström 2008 {published data only}
-
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. British Journal of Dermatology 2008;158(1):116-21. [CENTRAL: CN-00628309] [PMID: ] - PubMed
Gisondi 2008 {published data only}
-
- Gisondi P, Del Giglio M, Cotena C, Girolomoni G. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. British Journal of Dermatology 2008;158(6):1345-9. [CENTRAL: CN-00638916] [PMID: ] - PubMed
Goldfarb 1988 {published data only}
-
- Goldfarb MT, Ellis CN, Gupta AK, Tincoff T, Hamilton TA, Voorhees JJ. Acitretin improves psoriasis in a dose-dependent fashion. Journal of the American Academy of Dermatology 1988;18(4 Pt 1):655-62. [CENTRAL: CN-00053926] [PMID: ] - PubMed
-
- Gupta AK, Goldfarb MT, Ellis CN, Voorhees JJ. Side-effect profile of acitretin therapy in psoriasis. Journal of the American Academy of Dermatology 1989;20(6):1088-93. [CENTRAL: CN-00061373] [PMID: ] - PubMed
Gordon 2006 {published data only}
-
- Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. Journal of the American Academy of Dermatology 2006;55(4):598-606. [CENTRAL: CN-00568251] [PMID: ] - PubMed
-
- Shikiar R, Heffernan M, Langley RG, Willian MK, Okun MM, Revicki DA. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a phase II randomized controlled trial. Journal of Dermatological Treatment 2007;18(1):25-31. [CENTRAL: CN-00579275] [PMID: ] - PubMed
Gordon X‐PLORE 2015 {published data only}
-
- Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. New England Journal of Medicine 2015;373(2):136-44. [CENTRAL: CN-01076768] [PMID: ] - PubMed
Gottlieb 2003a {published data only}
-
- Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Archives of Dermatology 2003;139(12):1627-32. [CENTRAL: CN-00459604] [PMID: ] - PubMed
Gottlieb 2004a {published data only}
-
- Feldman SR, Gordon KB, Bala M, Evans R, Li S, Dooley LT, et al. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo-controlled trial. British Journal of Dermatology 2005;152(5):954-60. [CENTRAL: CN-00513174] [PMID: ] - PubMed
-
- Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. Journal of the American Academy of Dermatology 2004;51(4):534-42. [CENTRAL: CN-00501751] [PMID: ] - PubMed
Gottlieb 2011 {published data only}
-
- Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. British Journal of Dermatology 2011;165(3):652-60. [CENTRAL: CN-00811739] [PMID: ] - PubMed
Gottlieb 2012 {published data only}
-
- Gottlieb AB, Langley RG, Strober BE, Papp KA, Klekotka P, Creamer K, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. British Journal of Dermatology 2012;167(3):649-57. [CENTRAL: CN-00842357] [22533447] - PMC - PubMed
Gurel 2015 {published data only}
-
- Gurel G, Saracotlu ZN, Aksu AE. A single-blind study comparing acitretin and narrow-band UVB with the combination of placebo and narrow-band UVB in the treatment of plaque-type psoriasis [Plak tip psoriasis tedavisinde asitretin ve dar bant UVB ile plasebo ve dar bant UVB kombinasyonunun karsilastirilditi tek kor calisma]. Türkderm 2015;49(1):2-6. [CENTRAL: CN-01102298] [EMBASE: 2015064189]
Heydendael 2003 {published data only}
-
- Heydendael VM, Spuls PI, Opmeer BC, De Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. New England Journal of Medicine 2003;349(7):658-65. [CENTRAL: CN-00439969] [PMID: ] - PubMed
-
- Opmeer BC, Heydendael VM, De Borgie CA, Spuls PI, Bossuyt PM, Bos JD, et al. Costs of treatment in patients with moderate to severe plaque psoriasis: economic analysis in a randomized controlled comparison of methotrexate and cyclosporine. Archives of Dermatology 2004;140(6):685-90. [CENTRAL: CN-00468098] [PMID: ] - PubMed
Hunter 1963 {published data only}
-
- Hunter GA, Turner AN. Methotrexate in the treatment of psoriasis: a controlled clinical trial. Australasian Journal of Dermatology 1963;7(2):91-2. [CENTRAL: CN-01437408] [PMID: ] - PubMed
Igarashi 2012 {published data only}
-
- Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. Journal of Dermatology 2012;39(3):242-52. [CENTRAL: CN-00860708] [PMID: ] - PubMed
-
- Nakagawa H, Schenkel B, Kato M, Kato T, Igarashi A, Japanese Ustekinumab Study Group. Impact of ustekinumab on health-related quality of life in Japanese patients with moderate-to-severe plaque psoriasis: results from a randomized, double-blind, placebo-controlled phase 2/3 trial. Journal of Dermatology 2012;39(9):761-9. [CENTRAL: CN-00860068] [PMID: ] - PubMed
Ikonomidis 2017 {published data only}
-
- Ikonomidis I, Papadavid E, Makavos G, Andreadou I, Varoudi M, Gravanis K, et al. Lowering interleukin-12 activity improves myocardial and vascular function compared with tumor necrosis factor - a antagonism or cyclosporine in psoriasis. Circulation. Cardiovascular imaging 2017;10(9):e006283. [CENTRAL: CN-01412809] - PubMed
-
- Ikonomidis I, Varoudi M, Makavos G, Papadavid E, Kapniari I, Andreadou I, et al. Greater improvement of coronary artery function, left ventricular deformation and twisting by treatment with IL-17A antagonist compared to cyclosporine in psoriasis. European Heart Journal 2017;38(Suppl 1):688. [CENTRAL: CN-01468831]
-
- Ikonomidis I, Varoudi M, Makavos G, Papadavid E, Kapniari I, Andreadou I, et al. Treatment with IL-17A antagonist results in a greater improvement of coronary artery function, left ventricular deformation and twisting than cyclosporine in psoriasis. European Heart Journal - Cardiovascular Imaging 2017;18(Suppl 3):iii341. [CENTRAL: CN-01452103] [DOI: 10.1093/ehjci/jex298] - DOI
Ikonomidis 2022 {published data only}
IMMerge 2021 {published data only}
-
- NCT03478787. Risankizumab versus secukinumab for subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03478787 (first received 27 March 2018).
-
- Warren RB, Blauvelt A, Poulin Y, Beeck S, Kelly M, Wu T, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase 3, randomised, open-label, efficacy assessor-blinded clinical trial. British Journal of Dermatology 2021;184(1):50-9. [DOI: 10.1111/bjd.19341] - DOI - PMC - PubMed
IMMhance 2020 {unpublished data only}
-
- Blauvelt A, Papp K, Gooderham M, Langley RG, Leonardi C, Lacour JP, et al. Efficacy and safety of risankizumab, an IL-23 inihibitor in patients with moderate-to-severe chronic plaque psoriasis: 16-week phase 3 IMMhance trial results. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2018;16(Suppl 1):18. [CENTRAL: CN-01467570]
-
- Blauvelt A, Papp KA, Gooderham M, Langley RG, Leonardi C, Lacour JP, et al. Efficacy and safety of risankizumab, an interleukin-23 inhibitor, in patients with moderate-to-severe chronic plaque psoriasis: 16-week results from the phase III IMMhance trial. British Journal of Dermatology 2017;177(5):e248. [CENTRAL: CN-01452512]
-
- Blauvelt A, Papp KA, Gooderham M, Langley RG, Leonardi C, Lacour JP, et al. Risankizumab efficacy/safety in moderate-to-severe plaque psoriasis: 16-week results from IMMhance. Acta Dermato-Venereologica 2018;98(Suppl 219):30. [CENTRAL: CN-01620186]
-
- NCT02672852. BI 655066/ABBV-066 (Risankizumab) in moderate to severe plaque psoriasis with randomized withdrawal and re-treatment. clinicaltrials.gov/ct2/show/NCT02672852 (first received 1 February 2016). [CENTRAL: CN-01555522]
IMMpress 2022 {published data only}
-
- NCT03518047. Risankizumab therapy versus placebo for subjects with psoriasis in the Russian Federation (IMMPRESS). clinicaltrials.gov/show/nct03518047 (first received 8 May 2018).
IMMvent 2019 {unpublished data only}
-
- EUCTR2015-003623-65. BI 655066 (risankizumab) versus adalimumab in a randomised, double blind, parallel group trial in moderate to severe plaque psoriasis to assess safety and efficacy after 16 weeks of treatment and after inadequate adalimumab treatment response (IMMvent) - BI 655066 (risankizumab) versus adalimumab. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number%3A2... (first received 17 May 2016). [CENTRAL: CN-01855355]
-
- NCT02694523. BI 655066/ABBV-066 (risankizumab) compared to active comparator (adalimumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct02694523 (first received 29 February 2016). [CENTRAL: CN-01556126]
-
- Reich K, Gooderham M, Thaçi D, Crowley JJ, Ryan C, Krueger JG, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 2019;394(10198):576-86. [DOI: 10.1016/S0140-6736(19)30952-3] - DOI - PubMed
IXORA‐P 2018 {unpublished data only}
-
- Langley RG, Papp K, Gooderham M, Zhang L, Mallinckrodt C, Agada N, et al. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). British Journal of Dermatology 2018;178(6):1315-23. [CENTRAL: CN-01606242] - PubMed
-
- NCT02513550. A study comparing different dosing regimens of ixekizumab (LY2439821) in participants with moderate to severe plaque psoriasis (IXORA-P). clinicaltrials.gov/ct2/show/NCT02513550 (first received 30 July 2015). [CENTRAL: CN-01491284]
-
- Papp K, Orasan RI, Polzer P, Hennege C, Nica RD, Wilhelm S, et al. Absolute and relative pasi improvements with ixekizumab treatment: results at week 12 from IXORA-P. Acta Dermato-Venereologica 2018;98(Suppl 219):44-5. [CENTRAL: CN-01620199]
IXORA‐R 2020 {published data only}
-
- Blauvelt A, Leonardi C, Elewski B, Crowley JJ, Guenther LC, Gooderham M, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. British Journal of Dermatology 2020 Sep 2 [Epub ahead of print]. [DOI: 10.1111/bjd.19509] - DOI - PMC - PubMed
-
- Blauvelt A, Maari C, Gottlieb AB, ElMaraghy H, Young S, Lima RG, et al. 14152 Patient-reported outcomes in a head-to-head, randomized, double-blinded clinical trial of ixekizumab and guselkumab in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB19.
-
- Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety, and speed of response from a randomized, double-blinded trial. British Journal of Dermatology 2020;182(6):1348-58. - PMC - PubMed
-
- Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety, and speed of response from a randomized, double-blinded trial. British Journal of Dermatology 2020;182(6):1348-58. [DOI: 10.1111/bjd.18851] - DOI - PMC - PubMed
-
- Blauvelt A, Papp K, Jarel A, Reich K, Gottlieb AB, Lima RG, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate to severe plaque psoriasis: 12-week efficacy, safety, and speed of response from a randomized, double-blind trial. Journal of Clinical and Aesthetic Dermatology 2020;12(5 Suppl):S20-1.
IXORA‐S 2017 {unpublished data only}
-
- Anonymous. Rapid clinical response predicts consistent long-term response in patients with moderate-to-severe psoriasis: Ixekizumab vs. ustekinumab. Journal of the American Academy of Dermatology 2019;81(4 Suppl 1):AB113.
-
- Blauvelt A, Lomaga M, Burge R, Zhu B, Henneges C, Shen W, et al. Ixekizumab provides greater cumulative benefits versus ustekinumab over 24 weeks for patients with moderate-to-severe psoriasis in a randomized, double-blind phase 3b clinical trial. Acta Dermato-Venereologica 2018;98(Suppl 219):55-6. [CENTRAL: CN-01620173]
-
- Blauvelt A, Lomaga M, Burge R, Zhu B, Shen W, Shrom D, et al. Greater cumulative benefits from ixekizumab versus ustekinumab treatment over 52 weeks for patients with moderate-to-severe psoriasis in a randomized, double-blinded phase 3b clinical trial. Journal of Dermatological Treatment 2020;31(2):141-6. - PubMed
-
- Burge RT, Papadimitropoulos M, Henneges C, Garcia EG, Romiti R. Ixekizumab treatment leads to early resolution of bothersome symptoms versus ustekinumab. Value in Health 2017;20(9):A902. [CENTRAL: CN-01431388]
-
- Burkhardt N, Reich K, Lomaga M, Henneges C, Dossenbach M, Wilhelm S, et al. Efficacy and safety of ixekizumab (IXE) compared to ustekinumab (UST) in patients with moderate-to-severe plaque psoriasis: a randomised head-to-head trial. Australasian Journal of Dermatology 2017;58(Suppl 1):43. [CENTRAL: CN-01378805]
JUNCTURE 2015 {published data only}
-
- Lacour JP, Paul C, Jazayeri S, Papanastasiou P, Xu C, Nyirady J, et al. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. Journal of the European Academy of Dermatology and Venereology 2017;31(5):847-56. [CENTRAL: CN-01342022] [PMID: ] - PubMed
-
- Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). Journal of the European Academy of Dermatology and Venereology 2015;29(6):1082-90. [CENTRAL: CN-01043227] [PMID: ] - PubMed
Khatri 2016 {published data only}
-
- Khatri S, Amir Y, Min M, Goldblum O, Solotkin K, Yang F, et al. Early onset of clinical improvement with ixekizumab in patients with moderate-to-severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2016;30(S6):73-4. [CENTRAL: CN-01786974] [EMBASE: 611235569] - PMC - PubMed
-
- Khattri S, Goldblum O, Solotkin K, Amir Y, Min MS, Ridenour T, et al. Early onset of clinical improvement with ixekizumab in a randomized, open-label study of patients with moderate-to-severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5):33-7. [CENTRAL: CN-01610571] - PMC - PubMed
Krueger 2007 {published data only}
-
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. New England Journal of Medicine 2007;356(6):580-92. [CENTRAL: CN-00575216] [PMID: ] - PubMed
Laburte 1994 {published data only}
-
- Laburte C, Grossman R, Abi-Rached J, Abeywickrama KH, Dubertret L. Efficacy and safety of oral cyclosporin A (CyA; Sandimmun) for long-term treatment of chronic severe plaque psoriasis. British Journal of Dermatology 1994;130(3):366-75. [CENTRAL: CN-00100273] [PMID: ] - PubMed
Lee 2016 {published and unpublished data}
-
- Lee JH, Youn JI, Kim TY, Choi JH, Park CJ, Choe YB, et al. A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis. BMC Dermatology 2016;16(1):11. [CENTRAL: CN-01177229] [PMID: ] - PMC - PubMed
Leonardi 2003 {published data only}
-
- Feldman SR, Kimball AB, Krueger GG, Woolley JM, Lalla D, Jahreis A. Etanercept improves the health-related quality of life of patients with psoriasis: results of a phase III randomized clinical trial. Journal of the American Academy of Dermatology 2005;53(5):887-9. [CENTRAL: CN-00561361] [PMID: ] - PubMed
-
- Gordon KB, Gottlieb AB, Leonardi CL, Elewski BE, Wang A, Jahreis A, et al. Clinical response in psoriasis patients discontinued from and then reinitiated on etanercept therapy. Journal of Dermatological Treatment 2006;17(1):9-17 Erratum in: Journal of Dermatological Treatment 2006;17(3):192. [CENTRAL: CN-00555056] [PMID: ] - PubMed
-
- Krueger GG, Elewski B, Papp K, Wang A, Zitnik R, Jahreis A. Patients with psoriasis respond to continuous open-label etanercept treatment after initial incomplete response in a randomized, placebo-controlled trial. Journal of the American Academy of Dermatology 2006;54(3 Suppl 2):S112-9. [CENTRAL: CN-01625470] [PMID: ] - PubMed
-
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. New England Journal of Medicine 2003;349(21):2014-22. [CENTRAL: CN-00459025] [14627786] - PubMed
Leonardi 2012 {published data only}
-
- Edson-Heredia E, Banerjee S, Zhu B, Maeda-Chubachi T, Cameron GS, Shen W, et al. A high level of clinical response is associated with improved patient-reported outcomes in psoriasis: analyses from a phase 2 study in patients treated with ixekizumab. Journal of the European Academy of Dermatology and Venereology 2016;30(5):864-5. [CENTRAL: CN-01601237] [PMID: ] - PubMed
-
- Gordon KB, Leonardi CL, Lebwohl M, Blauvelt A, Cameron GS, Braun D, et al. A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2014;71(6):1176-82. [CENTRAL: CN-01091643] [PMID: ] - PubMed
-
- Langley RG, Rich P, Menter A, Krueger G, Goldblum O, Dutronc Y, et al. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2015;29(9):1763-70. [CENTRAL: CN-01089988] [PMID: ] - PubMed
-
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. New England Journal of Medicine 2012;366(13):1190-9. [CENTRAL: CN-00814008] [PMID: ] - PubMed
-
- Tham LS, Tang CC, Choi SL, Satterwhite JH, Cameron GS, Banerjee S. Population exposure-response model to support dosing evaluation of ixekizumab in patients with chronic plaque psoriasis. Journal of Clinical Pharmacology 2014;54(10):1117-24. [CENTRAL: CN-01048421] [PMID: ] - PubMed
LIBERATE 2017 {published data only}
-
- Reich K, Gooderham M, Bewley A, Green L, Soung J, Petric R, et al. Safety and efficacy of apremilast through 104 weeks in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment: findings from the LIBERATE study. Journal of the European Academy of Dermatology and Venereology 2018;32(3):397-402. [CENTRAL: CN-01643202] - PMC - PubMed
-
- Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). Journal of the European Academy of Dermatology and Venereology 2017;31(3):507-17. [CENTRAL: CN-01285623] [PMID: ] - PMC - PubMed
Liu 2020 {unpublished data only}
-
- Liu LF, Chen JS, Gu J, Xu JH, Jin HZ, Pang XW, et al. Etanercept biosimilar (recombinant human tumor necrosis factor-alpha receptor II: IgG Fc fusion protein) and methotrexate combination therapy in Chinese patients with moderate-to-severe plaque psoriasis: a multicentre, randomized, double-blind, placebo-controlled trial. Archives of Dermatological Research 2020;312(6):437-45. - PubMed
-
- NCT02313922. Optimizing psoriasis treatment of etanercept combined methotrexate. clinicaltrials.gov/ct2/show/NCT02313922 (first received 10 December 2014).
LOTUS 2013 {published data only}
-
- Zheng M, Zhu X-J, Song M, Shen Y-K, Wang B-X. A randomized, double-blind, placebo-controlled study of ustekinumab in Chinese patients with moderate to severe plaque psoriasis: LOTUS trial results. Journal of Dermatology 2012;39(s1):238-9. [CENTRAL: CN-01032271] [EMBASE: 70801108]
-
- Zhu X, Zheng M, Song M, Shen YK, Chan D, Szapary PO, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). Journal of Drugs in Dermatology 2013;12(2):166-74. [CENTRAL: CN-00965604] [PMID: ] - PubMed
-
- Zhu X-J, Zheng M, Song M, Han C, Chan D, Shen Y-K, et al. Ustekinumab improves health-related quality of life in Chinese patients with moderate-to-severe plaque psoriasis: results from the LOTUS trial and curative effect observation. Journal of Clinical Dermatology 2014;43(9):521-6. [CENTRAL: CN-01037236] [EMBASE: 2014949827]
Lowe 1991 {published data only}
-
- Lowe NJ, Prystowsky JH, Bourget T, Edelstein J, Nychay S, Armstrong R. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. Journal of the American Academy of Dermatology 1991;24(4):591-4. [CENTRAL: CN-00075422] [PMID: ] - PubMed
Mahajan 2010 {published data only}
-
- Mahajan R, Kaur I, Kanwar AJ. Methotrexate/narrowband UVB phototherapy combination vs. narrowband UVB phototherapy in the treatment of chronic plaque-type psoriasis - a randomized single-blinded placebo-controlled study. Journal of the European Academy of Dermatology and Venereology 2010;24(5):595-600. [CENTRAL: CN-00759274] [PMID: ] - PubMed
MATURE 2021 {published data only}
-
- EUCTR2018-000518-39-DE. Study of efficacy and safety of secukinumab 2 mL auto-injector (300 mg) injections in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-000518-39-DE (first received 15 November 2018).
-
- NCT03589885. Study of efficacy and safety of secukinumab 2 mL auto-injector (300 mg) in subjects with moderate to severe plaque psoriasis (MATURE). clinicaltrials.gov/show/nct03589885 (first received 18 July 2018).
-
- Sigurgeirsson B, Browning J, Tyring S, Szepietowski J C, Rivera-Diaz R, Effendy I, et al. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52-week results from MATURE, a randomized, placebo-controlled trial. Dermatologic Therapy 2022;35(3):e15285. - PubMed
-
- Sigurgeirsson B, Browning J, Tyring S, Szepietowski JC, Rivera-Diaz R, Effendy I, et al. 27599 Secukinumab administration by a 2 mL autoinjector demonstrates high efficacy with comparable safety and tolerability in adult patients with plaque psoriasis: 16-week results from MATURE. Journal of the American Academy of Dermatology 2021;85(3 Suppl):AB154.
-
- Sigurgeirsson B, Browning J, Tyring S, Szepietowski JC, Rivera-Diaz R, Effendy I, et al. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52-week results from MATURE, a randomized, placebo-controlled trial. Dermatolology Therapy 2022;35(3):e15285. [DOI: 10.1111/dth.15285] [PMID: ] - DOI - PubMed
Meffert 1997 {published data only}
-
- Meffert H, Bräutigam M, Färber L, Weidinger G. Low-dose (1.25 mg/kg) cyclosporin A: treatment of psoriasis and investigation of the influence on lipid profile. Acta Dermato-Venereologica 1997;77(2):137-41. [CENTRAL: CN-00138820] [PMID: ] - PubMed
METOP 2017 {published data only}
-
- Reich K, Reich JLK, Falk TM, Blödorn-Schlicht N, Mrowietz U, Von Kiedrowski R, et al. Clinical response of psoriasis to subcutaneous methotrexate correlates with inhibition of cutaneous T helper 1 and 17 inflammatory pathways. British Journal of Dermatology 2019;181(4):859-62. - PubMed
-
- Warren RB, Mrowietz U, Von Kiedrowski R, Niesmann J, Wilsmann-Theis D, Ghoreschi K, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389(10068):528-37. [CENTRAL: CN-01330160] [PMID: ] - PubMed
Morita 2022 {published data only}
Nakagawa 2016 {published data only}
-
- Nakagawa H, Niiro H, Ootaki K, Japanese Brodalumab Study Group. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. Journal of Dermatological Science 2016;81(1):44-52. [CENTRAL: CN-01133729] [PMID: ] - PubMed
-
- Umezawa Y, Nakagawa H, Niiro H, Ootaki K, Japanese Brodalumab Study Group. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2016;30(11):1957-60. [CENTRAL: CN-01457411] [PMID: ] - PubMed
NCT02134210 RaPsOdy {published data only}
-
- Kivitz AJ, Papp K, Devani A, Pinter A, Sinclair R, Ziv M, et al. Randomized, double-blind study comparing CHS-0214 with etanercept (ENBREL) in patients with psoriasis and psoriatic arthritis. Arthritis and Rheumatology 2016;68(Suppl 10):2142-3. [CENTRAL: CN-01292943]
-
- Leonardi C, Tang H, Kelleher C, Finck B. Evaluation of CHS-0214 as a proposed biosimilar to etanercept for the treatment of chronic plaque psoriasis: one-year results from a randomized, double-blind global trial. Journal of the American Academy of Dermatology 2017;76(6):AB128.
-
- NCT02134210. Comparison of CHS-0214 to Enbrel (etanercept) in patients with chronic plaque psoriasis (PsO). clinicaltrials.gov/show/nct02134210 (first received 9 May 2014). [CENTRAL: CN-01545599]
NCT02581345 {published data only}
-
- NCT02581345. Phase 3 study of M923 and Humira® in subjects with chronic plaque-type psoriasis. clinicaltrials.gov/show/nct02581345 (first received 21 October 2015). [CENTRAL: CN-01587601]
NCT02762994 {published data only}
-
- NCT02762994. International clinical trial to evaluate efficacy and safety of multiple subcutaneous injections of BCD-085 in various doses in patients with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02762994 (first received 5 May 2016).
NCT03055494 ObePso‐S {published data only}
-
- Krueger JG, Pariser D, Muscianisi E, Kianifard F, Steadman J, Prasad Sarkar MR, et al. 15354 Response to treatment with secukinumab in obese patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2020;83 (6 Suppl):AB38.
-
- Krueger JG, Pariser D, Tyring SK, Bagel J, Alexis AF, Soung J, et al. 15340 Long-term treatment with secukinumab led to sustained clinical improvement and normalization of inflammatory markers in patients with psoriasis. Journal of the American Academy of Dermatology 2020;83 (6 Suppl):AB37.
-
- NCT03055494. Study to explore the effect of secukinumab, compared to placebo, on fat tissue and skin in plaque psoriasis patients (ObePso-S). clinicaltrials.gov/show/nct03055494 (first received 16 February 2017).
NCT03364309 {published data only}
-
- NCT03364309. A study of ixekizumab (LY2439821) in Chinese participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/nct03364309 (first received 6 December 2017).
-
- Rui W, Li X, Zheng J, Pan W, Zheng M, Lu Y, et al. 25258 Efficacy and safety of ixekizumab in Chinese patients with moderate-to-severe plaque psoriasis: 12-week results from a phase 3 study. Journal of the American Academy of Dermatology 2021;85 (3 Suppl):AB55.
NCT03535194 OASIS‐2 {published data only}
-
- NCT03535194. A study to assess if mirikizumab is effective and safe compared to secukinumab and placebo in moderate to severe plaque psoriasis (OASIS-2). clinicaltrials.gov/show/nct03535194 (first received 24 May 2018).
-
- Papp K, Warren RB, Green LJ, Reich K, Langley R, Paul C, et al. Efficacy and safety of mirikizumab versus secukinumab and placebo in the treatment of moderate-to-severe psoriasis: 52-week results from OASIS-2, a multicenter, randomized, double-blind study. Journal of Clinical and Aesthetic Dermatology 2021;14 (5 Suppl):S26.
Nugteren‐Huying 1990 {published data only}
-
- Nugteren-Huying WM, Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy in psoriasis; a double-blind, placebo-controlled study [Fumaarzuurtherapie tegen psoriasis; een dubbelblind, placebo-gecontroleerd onderzoek]. Nederlands Tijdschrift voor Geneeskunde 1990;134(49):2387-91. [CENTRAL: CN-00072165] [PMID: ] - PubMed
-
- Nugteren-Huying WM, Van der Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy for psoriasis: a randomized, double-blind, placebo-controlled study. Journal of the American Academy of Dermatology 1990;22(2 Pt 1):311-2. [CENTRAL: CN-00066354] - PubMed
Ohtsuki 2017 {unpublished data only}
-
- NCT01988103. Efficacy and safety study of two doses of apremilast (CC-10004) in Japanese subjects with moderate-to-severe plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT01988103 (first received 24 May 2013). [CENTRAL: CN-01479115]
-
- Ohtsuki M, Okubo Y, Komine M, Imafuku S, Day RM, Chen P, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: efficacy, safety and tolerability results from a phase 2b randomized controlled trial. Journal of Dermatology 2017;44(8):873-84. [CENTRAL: CN-01600552] - PMC - PubMed
Ohtsuki 2018 {published data only}
-
- NCT02325219. An efficacy and safety of CNTO 1959 (Guselkumab) in participants with moderate to severe plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT02325219 (first received 24 December 2014).
-
- Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. Journal of Dermatology 2018;45(9):1053-62. [CENTRAL: CN-01646020] - PMC - PubMed
Olsen 1989 {published data only}
-
- Olsen EA, Weed WW, Meyer CJ, Cobo LM. A double-blind, placebo-controlled trial of acitretin for the treatment of psoriasis. Journal of the American Academy of Dermatology 1989;21(4 Pt 1):681-6. [CENTRAL: CN-00063370] [PMID: ] - PubMed
OPTIMAP 2022 {unpublished data only}
-
- Busard CI, Menting SP, Van Bezooijen JS, Van den Reek JM, Hutten BA, Prens EP, et al. Optimizing adalimumab treatment in psoriasis with concomitant methotrexate (OPTIMAP): study protocol for a pragmatic, single-blinded, investigator-initiated randomized controlled trial. Trials [Electronic Resource] 2017;18(1):52 Erratum in: Trials 2017; 18(1):113. - PMC - PubMed
-
- EUCTR2013-004918-18-NL. Optimising adalimumab treatment in psoriasis with concomitant methotrexate - OPTIMAP [Optimising adalimumab treatment in psoriasis with concomitant methotrexate]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-004918-18-NL/EUCT... (first received 12 December 2013).
-
- Van Der Kraaij G, Busard C, Van Den Reek J, Menting S, De Jong E, De Kort W, et al. Optimizing adalimumab treatment in psoriasis with concomitant methotrexate: a randomized controlled trial. Journal of the European Academy of Dermatology and Venereology 2019;33:25-6.
-
- Kraaij G, Busard C, den Reek J, Menting S, Musters A, Hutten B, et al. Adalimumab with methotrexate vs. adalimumab monotherapy in psoriasis: first-year results of a single-blind randomized controlled trial. Journal of investigative dermatology 2022;142(9):2375‐2383.e6. - PubMed
ORION 2020 {unpublished data only}
-
- Ferris L, Ott E, Hong HC, Baran W. Efficacy and safety of guselkumab administered with a novel self-injection device for the treatment of moderate-to-severe psoriasis: results from the phase III Orion self-dose study through week 16. Journal of the Dermatology Nurses' Association 2020;12(2):No pagination.
-
- Ferris L, Ott E, Jiang G, Chih-Ho Hong H, Baran W. Efficacy and safety of guselkumab administered with a novel self-injection device for the treatment of moderate-to-severe psoriasis: results from the phase III ORION self-dose study through week 16. Acta Dermato-Venereologica 2018;98(Suppl 219):29. [CENTRAL: CN-01620189]
-
- Ferris LK, Ott E, Jiang J, Hong HC, Li S, Han C, et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (One-Press), for moderate-to-severe psoriasis: results from the phase 3 ORION study. Journal of Dermatological Treatment 2020;31(2):152-9. - PubMed
-
- NCT02905331. Efficacy and safety study of guselkumab in the treatment of participants with moderate to severe plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT02905331 (first received 14 September 2016). [CENTRAL: CN-01520887]
Ortonne 2013 {published data only}
-
- Ortonne JP, Paul C, Berardesca E, Marino V, Gallo G, Brault Y, et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. British Journal of Dermatology 2013;168(5):1080-7. [CENTRAL: CN-00967538] [PMID: ] - PubMed
Papp 2005 {published data only}
-
- Krueger GG, Langley RG, Finlay AY, Griffiths CE, Woolley JM, Lalla D, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. British Journal of Dermatology 2005;153(6):1192-9. [CENTRAL: CN-00553127] [PMID: ] - PubMed
-
- Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. British Journal of Dermatology 2005;152(6):1304-12. [CENTRAL: CN-00522450] [PMID: ] - PubMed
Papp 2012a {published data only}
-
- Gordon KB, Kimball AB, Chau D, Viswanathan HN, Li J, Revicki DA, et al. Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory. British Journal of Dermatology 2014;170(3):705-15. [CENTRAL: CN-00981224] [PMID: ] - PMC - PubMed
-
- Papp K, Leonardi C, Menter A, Thompson EH, Milmont CE, Kricorian G, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. Journal of the American Academy of Dermatology 2014;71(6):1183-90.e3. [CENTRAL: CN-01107365] [EMBASE: 2015237135] - PubMed
-
- Papp K, Menter A, Strober B, Kricorian G, Thompson EH, Milmont CE, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2015;72(3):436-9.e1. [CENTRAL: CN-01111298] [PMID: ] - PubMed
-
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. New England Journal of Medicine 2012;366(13):1181-9. [CENTRAL: CN-00814009] [PMID: ] - PubMed
Papp 2012c {published data only}
-
- Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet 2012;380(9843):738-46. [CENTRAL: CN-00859723] [PMID: ] - PubMed
-
- Strand V, Fiorentino D, Hu C, Day RM, Stevens RM, Papp KA. Improvements in patient-reported outcomes with apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of moderate to severe psoriasis: results from a phase IIb randomized, controlled study. Health and Quality of Life Outcomes 2013;10(11):82. [CENTRAL: CN-00876574] [PMID: ] - PMC - PubMed
Papp 2013a {published data only}
-
- Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. British Journal of Dermatology 2013;168(2):412-21. [CENTRAL: CN-00967073] [PMID: ] - PubMed
-
- Sigurgeirsson B, Kircik L, Nemoto O, Mikazans I, Haemmerle S, Thurston HJ, et al. Secukinumab improves the signs and symptoms of moderate-to-severe plaque psoriasis in subjects with involvement of hands and/or feet: subanalysis of a randomized, double-blind, placebo-controlled, phase 2 dose-ranging study. Journal of the European Academy of Dermatology and Venereology 2014;28(8):1127-9. [CENTRAL: CN-01041806] [PMID: ] - PubMed
Papp 2013b {published data only}
-
- Papp KA, Kaufmann R, Thaçi D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. Journal of the European Academy of Dermatology and Venereology 2013;27(3):e376-83. [CENTRAL: CN-01124587] [PMID: ] - PubMed
Papp 2015 {published data only}
-
- Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. British Journal of Dermatology 2015;173(4):930-9. [CENTRAL: CN-01105188] [PMID: ] - PubMed
Papp 2017a {published data only}
-
- NCT01970488. Study to compare efficacy and safety of ABP 501 and adalimumab (HUMIRA®) in adults with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01970488 (first received 28 October 2013). [CENTRAL: CN-01478635]
-
- Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. Journal of the American Academy of Dermatology 2017;76(6):1093-102. [CENTRAL: CN-01401166] - PubMed
Papp 2017b {unpublished data only}
-
- NCT02054481. BI 655066 dose ranging in psoriasis, active comparator ustekinumab. clinicaltrials.gov/ct2/show/NCT02054481 (first received 3 February 2014).
-
- Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. New England Journal of Medicine 2017;376(16):1551-60. [CENTRAL: CN-01368160] - PubMed
Papp 2018 {published data only}
-
- Catlett IM, Hu S, Banerjee S, Gordon K, Krueger JG. A selective inhibitor of TYK2, BMS-986165, improves molecular, cellular, and clinical biomarkers associated with efficacy in moderate-to-severe psoriasis. Experimental Dermatology 2018;27(Suppl 2):55. [CENTRAL: CN-01791579]
-
- Catlett IM, Hu S, Banerjee S, Gordon K, Krueger JG. A selective inhibitor of tyrosine kinase 2, bms-986165, improves molecular, cellular, and clinical biomarkers associated with efficacy in moderate-to-severe psoriasis. Journal of the Dermatology Nurses' Association (Conference: 24th World Congress of Dermatology, Italy) 2020;12(2):No pagination.
-
- EUCTR2016-002481-31-LV. Study to evaluate effectiveness and safety in subjects with moderate to severe psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-002481-31-LV (first received 28 November 2016).
-
- Gooderham M, Papp K, Gordon K, Foley P, Morita A, Thaçi D, et al. Influence of baseline demographics on efficacy of a selective oral TYK2 inhibitor, BMS-986165, in patients with moderate-to-severe plaque psoriasis: a Phase 2, randomized, placebo-controlled trial. Experimental Dermatology 2018;27(Suppl 2):32. [CENTRAL: CN-01791582]
-
- Gordon K, Papp K, Gooderham M, Thaçi D, Foley P, Morita A, et al. Evaluating influence of baseline characteristics on efficacy of a selective oral TYK2 inhibitor, BMS-986165, in patients with moderate-to-severe plaque psoriasis in a phase 2 trial. Experimental Dermatology 2018;27(Suppl 2):29-30. [CENTRAL: CN-01787103]
Papp 2021 {published data only}
-
- NCT03384745. A phase 2b study of the efficacy, safety, and tolerability of M1095 in subjects with moderate to severe psoriasis. clinicaltrials.gov/show/nct03384745 (first received 27 December 2017).
-
- Papp KA, Weinberg MA, Morris A, Reich K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomised, placebo-controlled, phase 2b study. Lancet 2021;397(10284):1564-75 Erratum in Lancet 2021; 397(10290):2150. - PubMed
PEARL 2011 {published data only}
-
- Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). Journal of Dermatological Science 2011;63(3):154-63. [CENTRAL: CN-00810821] [PMID: ] - PubMed
-
- Tsai TF, Song M, Shen YK, Schenkel B, Choe YB, Kim NI, et al. Ustekinumab improves health-related quality of life in Korean and Taiwanese patients with moderate to severe psoriasis: results from the PEARL trial. Journal of Drugs in Dermatology 2012;11(8):943-9. [CENTRAL: CN-00859638] [PMID: ] - PubMed
PHOENIX‐1 2008 {published data only}
-
- Guenther L, Han C, Szapary P, Schenkel B, Poulin Y, Bourcier M, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. Journal of the European Academy of Dermatology and Venereology 2011;25(7):851-7. [CENTRAL: CN-00887488] [EMBASE: 2011360521] - PubMed
-
- Hu C, Szapary PO, Yeilding N, Zhou H. Informative dropout modeling of longitudinal ordered categorical data and model validation: application to exposure-response modeling of physician's global assessment score for ustekinumab in patients with psoriasis. Journal of Pharmacokinetics and Pharmacodynamics 2011;38(2):237-60. [CENTRAL: CN-00787444] [PMID: ] - PubMed
-
- Kimball AB, Gordon KB, Fakharzadeh S, Yeilding N, Szapary PO, Schenkel B, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. British Journal of Dermatology 2012;166(4):861-72. [CENTRAL: CN-00841277] [PMID: ] - PubMed
-
- Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. Journal of the European Academy of Dermatology and Venereology 2013;27(12):1535-45. [CENTRAL: CN-00915003] [PMID: ] - PubMed
-
- Lebwohl M, Leonardi C, Griffiths CE, Prinz JC, Szapary PO, Yeilding N, et al. Long-term safety experience of ustekinumab in patients with moderate-to-severe psoriasis (part I of II): results from analyses of general safety parameters from pooled phase 2 and 3 clinical trials. Journal of the American Academy of Dermatology 2012;66(5):731-41. [CENTRAL: CN-00860736] [PMID: ] - PubMed
PHOENIX‐2 2008 {published data only}
-
- Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu MC, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. Journal of the American Academy of Dermatology 2010;63(3):457-65. [CENTRAL: CN-00761719] [PMID: ] - PubMed
-
- Langley RG, Lebwohl M, Krueger GG, Szapary PO, Wasfi Y, Chan D, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. British Journal of Dermatology 2015;172(5):1371-83. [CENTRAL: CN-01254538] [PMID: ] - PubMed
-
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008;371(9625):1675-84. [CENTRAL: CN-00631486] [PMID: ] - PubMed
-
- Reich K, Schenkel B, Zhao N, Szapary P, Augustin M, Bourcier M, et al. Ustekinumab decreases work limitations, improves work productivity, and reduces work days missed in patients with moderate-to-severe psoriasis: results from PHOENIX 2. Journal of Dermatological Treatment 2011;22(6):337-47. [CENTRAL: CN-00860939] [PMID: ] - PubMed
PIECE 2016 {published data only}
-
- De Vries AC, Thio HB, De Kort WJ, Opmeer BC, Van der Stok HM, De Jong EM, et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. British Journal of Dermatology 2016;176(3):624-33. [CENTRAL: CN-01336979] [PMID: ] - PubMed
Piskin 2003 {published data only}
-
- Piskin G, Heydendael VM, Rie MA, Bos JD, Teunissen MB. Cyclosporin A and methotrexate are equally effective in reducing T cell numbers in psoriatic skin lesions but have no consistent effect on IFN-gamma and IL-4 expression in psoriatic skin in situ. Archives of Dermatological Research 2003;294(12):559-62. [CENTRAL: CN-00456554] [PMID: ] - PubMed
PLANETA 2021 {published data only}
-
- Andrey B, Aleksey S, Antonina A, Ekaterina C, Roman I. Netakimab: 12-week results from Planeta study, a phase III trial of a novel il-17 inhibitor in moderate-to-severe plaque psoriasis. Journal of the Dermatology Nurses' Association 2020;12(2):No pagination.
-
- Puig L, Bakulev AL, Kokhan MM, Samtsov AV, Khairutdinov VR, Morozova MA, et al. Efficacy and safety of netakimab, a novel anti-IL-17 monoclonal antibody, in patients with moderate to severe plaque psoriasis. Results of a 54-week randomized double-blind placebo-controlled PLANETA clinical trial. Dermatology and Therapy 2021;11(4):1319-32. - PMC - PubMed
-
- Puig L, Bakulev AL, Kokhan MM, Samtsov AV, Khairutdinov VR, Morozova MA, et al. Efficacy and safety of netakimab, a novel anti-IL-17 monoclonal antibody, in patients with moderate to severe plaque psoriasis. Results of a 54-week randomized double-blind placebo-controlled PLANETA clinical trial. Dermatology and Therapy 2021;31:31. - PMC - PubMed
POETYK PSO‐1 2022 {published data only}
-
- Armstrong AW, Gooderham M, Warren RB, Papp K, Strober B, Thaci D, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. Journal of the American Academy of Dermatology 2022 Jul 9 [Epub ahead of print]. [DOI: 10.1016/j.jaad.2022.07.002] [PMID: ] - DOI - PubMed
-
- Armstrong AW, Gooderham M, Warren RB, Papp K, Strober B, Thaci D, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the phase 3 POETYK PSO-1 study. Annals of the Rheumatic Diseases 2021;80(Suppl 1):795-6.
-
- Augustin M, Strober B, Armstrong A W, Beaumont J, Pham T P, Hudgens S, Zhuo J, Becker B, Banerjee S, Kisa R M, Papp K. 35205 Deucravacitinib improves Dermatology Life Quality Index (DLQI) in patients with moderate to severe psoriasis: results from the phase 3 POETYK PSO-1 and PSO-2 trials. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB156.
-
- EUCTR2018-001926-25-ES. Efficacy and safety of BMS-986165 versus placebo and active comparator in subjects with psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001926-25-ES (first received 25 October 2018).
-
- Foley P. Deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, versus placebo and apremilast in psoriasis: efficacy analysis by prior treatment in the phase 3 POETYK PSO-1 and PSO-2 trials. Australasian Journal of Dermatology 2022;63(Suppl 1):65.
POETYK PSO‐2 2022 {published data only}
-
- NCT03611751. An investigational study to evaluate experimental medication BMS-986165 compared to placebo and a currently available treatment in participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/nct03611751 (first received 2 August 2018).
-
- Strober B, Thaci D, Sofen H, Kircik L, Gordon KB, Foley P, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 POETYK PSO-2 trial. Journal of the American Academy of Dermatology 2022;14:14. - PubMed
POETYK PSO‐3 2022 {published data only}
-
- NCT04167462. An investigational study to evaluate experimental medication BMS-986165 compared to placebo in participants with plaque psoriasis in mainland China, Taiwan, and South Korea (POETYK-PSO-3). clinicaltrials.gov/show/NCT04167462 (first received 18 November 2019).
POLARIS 2020 {unpublished data only}
-
- NCT02951533. A study to compare the efficacy of guselkumab to fumaric acid esters for the treatment of participants with moderate to severe plaque psoriasis (POLARIS). clinicaltrials.gov/ct2/show/NCT02951533 (first received 28 October 2016). [CENTRAL: CN-01559701]
-
- Thaçi D, Pinter A, Sebastian M, Termeer C, Sticherling M, Gerdes S, et al. Guselkumab is superior to fumaric acid esters in patients with moderate-to-severe plaque psoriasis who are naïve to systemic treatment: results from a randomised, active comparator-controlled phase 3b trial (POLARIS). British Journal of Dermatology 2020;183(2):265-75. [DOI: 10.1111/bjd.18696] - DOI - PubMed
PRESTA 2010 {published data only}
-
- Damjanov N, Karpati S, Kemeny L, Bakos N, Bobic B, Majdan M, et al. Efficacy and safety of etanercept in psoriasis and psoriatic arthritis in the PRESTA study: analysis in patients from Central and Eastern Europe. Journal of Dermatological Treatment 2018;29(1):8-12. [CENTRAL: CN-01458628] - PubMed
-
- Gniadecki R, Robertson D, Molta CT, Freundlich B, Pedersen R, Li W, et al. Self-reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. Journal of the European Academy of Dermatology and Venereology 2012;26(11):1436-43. [CENTRAL: CN-00971660] [PMID: ] - PubMed
-
- Griffiths CE, Sterry W, Brock F, Dilleen M, Stefanidis D, Germain JM, et al. Pattern of response in patients with moderate-to-severe psoriasis treated with etanercept. British Journal of Dermatology 2015;172(1):230-8. [CENTRAL: CN-01116415] [PMID: ] - PubMed
-
- Kirkham B, De Vlam K, Li W, Boggs R, Mallbris L, Nab HW, et al. Early treatment of psoriatic arthritis is associated with improved patient-reported outcomes: findings from the etanercept PRESTA trial. Clinical and Experimental Rheumatology 2015;33(1):11-9. [CENTRAL: CN-01090489] [PMID: ] - PubMed
-
- Prinz JC, Fitzgerald O, Boggs RI, Foehl J, Robertson D, Pedersen R, et al. Combination of skin, joint and quality of life outcomes with etanercept in psoriasis and psoriatic arthritis in the PRESTA trial. Journal of the European Academy of Dermatology and Venereology 2011;25(5):559-64. [CENTRAL: CN-00802619] [PMID: ] - PubMed
PRIME 2017 {unpublished data only}
-
- NCT02474082. Study of secukinumab compared to fumaderm® in adults with moderate to severe psoriasis (PRIME). clinicaltrials.gov/ct2/show/NCT02474082 (first received 16 April 2015). [CENTRAL: CN-01552941]
-
- Sticherling M, Mrowietz U, Augustin M, Thaçi D, Melzer N, Hentschke C, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. British Journal of Dermatology 2017;177(4):1024-32 Corrigendum to British Journal of Dermatology 2017; 177(6):1772. [CENTRAL: CN-01427286] - PubMed
PRISTINE 2013 {published data only}
-
- Puig L, Strohal R, Husni ME, Tsai TF, Noppakun N, Szumski A, et al. Cardiometabolic profile, clinical features, quality of life and treatment outcomes in patients with moderate-to-severe psoriasis and psoriatic arthritis. Journal of Dermatological Treatment 2015;26(1):7-15. [CENTRAL: CN-01051703] [PMID: ] - PubMed
-
- Strohal R, Puig L, Chouela E, Tsai TF, Melin J, Freundlich B, et al. The efficacy and safety of etanercept when used with as-needed adjunctive topical therapy in a randomised, double-blind study in subjects with moderate-to-severe psoriasis (the PRISTINE trial). Journal of Dermatological Treatment 2013;24(3):169-78. [CENTRAL: CN-00881482] [PMID: ] - PubMed
-
- Thaçi D, Galimberti R, Amaya-Guerra M, Rosenbach T, Robertson D, Pedersen R, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. Journal of the European Academy of Dermatology and Venereology 2014;28(7):900-6. [CENTRAL: CN-01041533] [PMID: ] - PubMed
PsOsim 2017 {published data only}
-
- EUCTR2015-000632-15-EE. A study to compare the efficacy and safety of CHS-1420 against Humira®. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-000632-15-EE (first received 22 September 2015).
-
- Hodge J, Tang H, O'Connor P, Finck B. Switching from adalimumab to CHS-1420: a randomized, double-blind global clinical trial in patients with psoriasis and psoriatic arthritis. Arthritis and Rheumatology 2017;69(Suppl 10):2879.
-
- NCT02489227. Comparison of CHS-1420 versus Humira in subjects with chronic plaque psoriasis (PsOsim). clinicaltrials.gov/show/nct02489227 (first received 2 July 2015).
Rathipriyadharshini 2020 {published data only}
-
- CTRI/2019/01/017362. A study to assess the effects of apremilast and methotrexate in the treatment of patients with psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/01/017362 (first received 31 January 2019).
-
- Rathipriyadharshini R, Prem Kumar M, Soundarya S, Srinivasan MS. An open-labelled randomised comparative evaluation of therapeutic efficacy and safety of apremilast versus methotrexate in the treatment of patients with chronic plaque psoriasis. Annals of Tropical Medicine and Public Health 2020;23(15):231517.
Reich 2012a {published data only}
-
- Reich K, Ortonne JP, Gottlieb AB, Terpstra IJ, Coteur G, Tasset C, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab' certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. British Journal of Dermatology 2012;167(1):180-90. [CENTRAL: CN-00856435] [PMID: ] - PubMed
Reich 2015 {published data only}
Reich 2020 {unpublished data only}
-
- Leutz A, Pinter A, Thaci D, Augustin M, Schuster C, Fotiou K, et al. Efficacy and safety of ixekizumab after switching from fumaric acid esters or methotrexate in patients with moderate-to-severe plaque psoriasis naive to systemic treatment. British Journal of Dermatology 2021;184(3):548-50. - PubMed
-
- NCT02634801. A study of Ixekizumab (LY2439821) in participants with moderate-to-severe plaque psoriasis naive to systemic treatment. clinicaltrials.gov/ct2/show/NCT02325219 (first received 16 December 2015). [CENTRAL: CN-01554547]
-
- Reich K, Augustin M, Thaci D, Pinter A, Leutz A, Henneges C, et al. A 24-week multicentre, randomised, open-label, parallel-group study comparing the efficacy and safety of ixekizumab to fumaric acid esters and methotrexate in patients with moderate-to-severe plaque psoriasis naïve to systemic treatment. British Journal of Dermatology 2020;182(4):869-79. [DOI: 10.1111/bjd.18384] - DOI - PMC - PubMed
ReSURFACE‐1 2017 {published data only}
-
- Anonymous. Erratum: Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials (Lancet 2017; 390(10091):276-288). Lancet 2017;390(10091):230. - PubMed
-
- Blauvelt A, Sofen H, Papp K, Gooderham M, Tyring S, Zhao Y, et al. Tildrakizumab efficacy and impact on quality of life up to 52 weeks in patients with moderate-to-severe psoriasis: a pooled analysis of two randomized controlled trials. Journal of the European Academy of Dermatology and Venereology 2019;33(12):2305-12. [DOI: 10.1111/jdv.15862] - DOI - PMC - PubMed
-
- NCT01722331. A study to evaluate the efficacy and safety of subcutaneous MK-3222, followed by an optional long-term safety extension study, in participants with moderate-to-severe chronic plaque psoriasis (MK-3222-010). clinicaltrials.gov/ct2/show/NCT01722331 (first received 16 March 2017).
-
- Reich K, Blauvelt A, Thaçi D, Papp KA, Kimball A, Sinclair R, et al. Safety and tolerability of tildrakizumab in patients with chronic plaque psoriasis: esults from long-term extensions of 2 phase 3 studies. Australasian Journal of Dermatology 2018;59(Suppl 1):110-1. [CENTRAL: CN-01606961]
-
- Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390(10091):276-88. [CENTRAL: CN-01422560] - PubMed
ReSURFACE‐2 2017 {unpublished data only}
-
- NCT01729754. A study to evaluate the efficacy and safety/tolerability of subcutaneous tildrakizumab (SCH 900222/MK-3222) in participants with moderate-to-severe chronic plaque psoriasis followed by a long-term extension study (MK-3222-011). clinicaltrials.gov/ct2/show/NCT01729754 (first received 13 November 2012). [CENTRAL: CN-01538777]
-
- Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390(10091):276-88. [CENTRAL: CN-01422560] - PubMed
REVEAL 2008 {published data only}
-
- Armstrong AW, Villanueva Quintero DG, Echeverria CM, Gu Y, Karunaratne M, Reyes Servin O. Body region involvement and quality of life in psoriasis: analysis of a randomized controlled trial of adalimumab. American Journal of Clinical Dermatology 2016;17(6):691-9. [CENTRAL: CN-01286209] [PMID: ] - PMC - PubMed
-
- Gordon K, Papp K, Poulin Y, Gu Y, Rozzo S, Sasso EH. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. Journal of the American Academy of Dermatology 2012;66(2):241-51. [CENTRAL: CN-00860821] [PMID: ] - PubMed
-
- Kimball AB, Bensimon AG, Guerin A, Yu AP, Wu EQ, Okun MM, et al. Efficacy and safety of adalimumab among patients with moderate to severe psoriasis with co-morbidities: Subanalysis of results from a randomized, double-blind, placebo-controlled, phase III trial. American Journal of Clinical Dermatology 2011;12(1):51-62. [CENTRAL: CN-00779604] [PMID: ] - PubMed
-
- Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. Journal of the American Academy of Dermatology 2010;62(5):812-8. [CENTRAL: CN-00743358] [PMID: ] - PubMed
-
- Menter A, Gordon KB, Leonardi CL, Gu Y, Goldblum OM. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. Journal of the American Academy of Dermatology 2010;63(3):448-56. [CENTRAL: CN-00761638] [PMID: ] - PubMed
Rich 2013 {published data only}
-
- Augustin M, Abeysinghe S, Mallya U, Qureshi A, Roskell N, McBride D, et al. Secukinumab treatment of plaque psoriasis shows early improvement in DLQI response - results of a phase II regimen-finding trial. Journal of the European Academy of Dermatology and Venereology 2016;30(4):645-9. [CENTRAL: CN-01265142] [PMID: ] - PMC - PubMed
-
- Paul C, Reich K, Gottlieb AB, Mrowietz U, Philipp S, Nakayama J, et al. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. Journal of the European Academy of Dermatology and Venereology 2014;28(12):1670-5. [CENTRAL: CN-01119275] [PMID: ] - PubMed
-
- Rich P, Sigurgeirsson B, Thaçi D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. British Journal of Dermatology 2013;168(2):402-11. [CENTRAL: CN-00965685] [PMID: ] - PubMed
Ruzicka 1990 {published data only}
-
- Ruzicka T, Sommerburg C, Braun-Falco O, Köster W, Lengen W, Lensing W, et al. Efficiency of acitretin in combination with UV-B in the treatment of severe psoriasis. Archives of Dermatology 1990;126(4):482-6. [CENTRAL: CN-00066767] [PMID: ] - PubMed
Sandhu 2003 {published data only}
-
- Sandhu K, Kaur I, Kumar B, Saraswat A. Efficacy and safety of cyclosporine versus methotrexate in severe psoriasis: a study from North India. Journal of Dermatology 2003;30(6):458-63. [CENTRAL: CN-00456950] [PMID: ] - PubMed
Saurat 1988 {published data only}
-
- Saurat JH, Geiger JM, Amblard P, Beani JC, Boulanger A, Claudy A, et al. Randomized double-blind multicenter study comparing acitretin-PUVA, etritinate-PUVA and placebo-PUVA in the treatment of severe psoriasis. Dermatologica 1988;177(4):218-24. [CENTRAL: CN-00058056] [PMID: ] - PubMed
SCULPTURE 2015 {published data only}
-
- Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). Journal of the American Academy of Dermatology 2015;73(1):27-36.e1. [CENTRAL: CN-01109352] [PMID: ] - PubMed
Seo 2020 {published data only}
-
- NCT02982005. A study of KHK4827 (brodalumab) in subjects with moderate to severe psoriasis in Korea. clinicaltrials.gov/ct2/show/NCT02982005 (first received 5 December 2016).
Shehzad 2004 {published data only}
-
- Shehzad T, Dar NR, Zakria M. Efficacy of concomitant use of puva and methotrexate in disease clearance time in plaque type psoriasis. Journal of the Pakistan Medical Association 2004;54(9):453-5. [CENTRAL: CN-00727152] [PMID: ] - PubMed
SIGNATURE 2019 {unpublished data only}
-
- NCT01961609. Secukinumab in TNF-IR psoriasis patients (SIGNATURE). clinicaltrials.gov/ct2/show/NCT01961609 (first received 10 October 2013). [CENTRAL: CN-01536745]
-
- Warren RB, Barker JNWB, Finlay AY, Burden AD, Kirby B, Armendariz Y, et al. Secukinumab for patients failing previous tumour necrosis factor-alpha inhibitor therapy: results of a randomized open-label study (SIGNATURE). British Journal of Dermatology 2019;183(1):60-70. [DOI: 10.1111/bjd.18623] - DOI - PubMed
Singh 2021 {published data only}
-
- Singh SK, Singnarpi SR. Safety and efficacy of methotrexate (0.3 mg/kg/week) versus a combination of methotrexate (0.15 mg/kg/week) with cyclosporine (2.5 mg/kg/day) in chronic plaque psoriasis: a randomised non-blinded controlled trial. Indian Journal of Dermatology, Venereology and Leprology 2021;87(2):214-22. - PubMed
Sommerburg 1993 {published data only}
-
- Sommerburg C, Kietzmann H, Eichelberg D, Goos M, Heese A, Holzle E, et al. Acitretin in combination with PUVA: a randomized double-blind placebo-controlled study in severe psoriasis. Journal of the European Academy of Dermatology and Venereology 1993;2(4):308-17. [CENTRAL: CN-00180920] [EMBASE: 1993350796]
SPIRIT‐H2H 2020 {published data only}
-
- CTRI/2017/09/009850. Comparison of ixekizumab with adalimumab in patients with psoriatic arthritis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/09/009850 (first received 19 September 2017).
-
- Edwards CJ, Bradley A, Nassab MH, Moller B, Machold KP, Sapin C, et al. Ixekizumab vs. adalimumab for the treatment of psoriatic arthritis: 52-week efficacy and safety outcomes. Swiss Medical Weekly 2020;150(Suppl 245):7S.
-
- Edwards CJ, Bradley AJ, Nassab MH, Moller B, Machold KP, Sapin C, et al. Ixekizumab (IXE) vs. adalimumab (ADA) for the treatment of PSA: 52-week efficacy and safety outcomes. Rheumatology (United Kingdom) 2020;59 (Suppl 2):ii10-1.
-
- Kavanaugh A, Lubrano E, Muram T, Lin CY, Liu Leage S, Van Den Bosch F, et al. Head-to-head study evaluating the combined ACR50/PASI100 treatment response of ixekizumab versus adalimumab: individual patient data from a randomized, openlabel study in biologic-naive patients with psoriatic arthritis through week 52. Annals of the Rheumatic Diseases 2020;79(Suppl 1):767.
-
- Kleyn CE, Gooderham M, Kristensen LE, De Vlam K, Schuster C, Liu Leage S, et al. Efficacy of ixekizumab vs. adalimumab in patients with psoriatic arthritis with moderate-to-severe psoriasis: 52-week results from a multicentre, randomized, open-label study. British Journal of Dermatology 2020;183 (Suppl 1):70-1.
Strober 2011 {published data only}
-
- Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. British Journal of Dermatology 2011;165(3):661-8. [CENTRAL: CN-00811738] [PMID: ] - PubMed
STYLE 2020 {published data only}
-
- Van Voorhees AS, Stein Gold L, Lebwohl M, Strober B, Lynde C, Tyring S, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. Journal of the American Academy of Dermatology 2020;83(1):96-103. - PubMed
SustaIMM 2019 {published data only}
-
- NCT03000075. BI 655066 (risankizumab) compared to placebo in Japanese patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct03000075 (first received 21 December 2016). [CENTRAL: CN-01560779]
Tanew 1991 {published data only}
-
- Tanew A, Guggenbichler A, Hönigsmann H, Geiger JM, Fritsch P. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. Journal of the American Academy of Dermatology 1991;25(4):682-4. [CENTRAL: CN-00612571] [PMID: ] - PubMed
Thaci 2021 {published data only}
-
- NCT03255382. A study to assess the efficacy of risankizumab compared to FUMADERM® in subjects with moderate to severe plaque psoriasis who are naive to and candidates for systemic therapy. clinicaltrials.gov/show/nct03255382 (first received 21 August 2017).
-
- Thaci D, Eyerich K, Pinter A, Sebastian M, Unnebrink K, Rubant S, et al. Direct comparison of risankizumab and fumaric acid esters in systemic therapy-naive patients with moderate-to-severe plaque psoriasis: a randomized controlled trial. British Journal of Dermatology 2022;186(1):30-9. - PMC - PubMed
-
- Thaci D, Eyerich K, Pinter A, Sebastian M, Unnebrink K, Rubant S, et al. Direct comparison of risankizumab and fumaric acid esters in systemic-therapy-naive patients with moderate to severe plaque psoriasis: a randomized controlled trial. British Journal of Dermatology 2021;15(186):30-9. - PMC - PubMed
-
- Thaci D, Soliman AM, Eyerich K, Pinter A, Sebastian M, Unnebrink K, et al. Patient-reported outcomes with risankizumab versus fumaric acid esters in systemic therapy-naive patients with moderate to severe plaque psoriasis: a phase 3 clinical trial. Journal of the European Academy of Dermatology and Venereology 2021;35(8):1686-91. - PubMed
Torii 2010 {published data only}
-
- JPRN-JapicCTI-060318. Clinical study to evaluate the efficacy and safety of TA-650 in patients with psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-060318 (first received 1 November 2006).
-
- Torii H, Nakagawa H, Japanese Infliximab Study investigators. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. Journal of Dermatological Science 2010;59(1):40-9. [CENTRAL: CN-00760986] [PMID: ] - PubMed
TRANSFIGURE 2016 {published and unpublished data}
-
- Reich K, Sullivan J, Arenberger P, Mrowietz U, Jazayeri S, Augustin M, et al. Secukinumab shows significant efficacy in nail psoriasis: week 32 results from the TRANSFIGURE study. Annals of the Rheumatic Diseases 2016;75(Suppl 2):603-4. [CENTRAL: CN-01761193]
Tyring 2006 {published data only}
-
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. British Journal of Dermatology 2007;157(6):1275-7. [CENTRAL: CN-00627972] [PMID: ] - PubMed
-
- Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EH, Gandra SR, et al. Patient-reported outcomes in moderate-to-severe plaque psoriasis with scalp involvement: results from a randomized, double-blind, placebo-controlled study of etanercept. Journal of the European Academy of Dermatology and Venereology 2013;27(1):125-8. [CENTRAL: CN-00971136] [PMID: ] - PubMed
-
- Tyring S, Gordon KB, Poulin Y, Langley RG, Gottlieb AB, Dunn M, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Archives of Dermatology 2007;143(6):719-26. [CENTRAL: CN-00589825] [PMID: ] - PubMed
-
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006;367(9504):29-35. [CENTRAL: CN-00532672] [PMID: ] - PubMed
UltIMMa‐1 2018 {unpublished data only}
-
- Gooderham M, Lebwohl M, Gordon K, Wu T, Photowala H, Bachelez H. High and durable clearance through 52 weeks of risankizumab treatment in patients with moderate-to-severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2019;33(S3):20.
-
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392(10148):650-61. [CENTRAL: CN-01649259] - PubMed
-
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab: results from two double-blind, placebo-and ustekinumab-controlled, phase 3 trials in moderate-to-severe plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):28-9. - PubMed
-
- NCT02684357. BI 655066 compared to placebo & active comparator (Ustekinumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02684357 (first received 18 February 2016). [CENTRAL: CN-01555862]
UltIMMa‐2 2018 {unpublished data only}
-
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018;392(10148):650-61. [CENTRAL: CN-01649259] - PubMed
-
- Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab: results from two double-blind, placebo-and ustekinumab-controlled, phase 3 trials in moderate-to-severe plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):28-9. - PubMed
-
- NCT02684370. BI 655066 (Risankizumab) compared to placebo and active comparator (Ustekinumab) in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/ct2/show/NCT02684370 (first received 18 February 2016). [CENTRAL: CN-01555863]
Umezawa 2021 {published data only}
-
- NCT03051217. A study to test the efficacy and safety of certolizumab pegol in Japanese subjects with moderate to severe chronic psoriasis. clinicaltrials.gov/show/nct03051217 (first received 13 February 2017).
UNCOVER‐1 2016 {published data only}
-
- Armstrong AW, Lynde CW, McBride SR, Stahle M, Edson-Heredia E, Zhu B, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatology 2016;152(6):661-9. [CENTRAL: CN-01166284] [PMID: ] - PubMed
-
- Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New England Journal of Medicine 2016;375(4):345-56. [CENTRAL: CN-01167902] [PMID: ] - PubMed
UNCOVER‐2 2015 {published data only}
-
- Griffiths CE, Reich K, Lebwohl M, Van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386(9993):541-51. [CENTRAL: CN-01091029] [PMID: ] - PubMed
UNCOVER‐3 2015 {published data only}
-
- Griffiths CE, Reich K, Lebwohl M, Van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386(9993):541-51. [CENTRAL: CN-01091029] [PMID: ] - PubMed
-
- Van de Kerkhof P, Guenther L, Gottlieb AB, Sebastian M, Wu JJ, Foley P, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. Journal of the European Academy of Dermatology and Venereology 2017;31(3):477-82. [CENTRAL: CN-01287590] [PMID: ] - PubMed
Van Bezooijen 2016 {published data only}
Van de Kerkhof 2008 {published data only}
-
- Reich K, Segaert S, Van de Kerkhof P, Durian C, Boussuge MP, Paolozzi L, et al. Once-weekly administration of etanercept 50 mg improves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology (Basel, Switzerland) 2009;219(3):239-49. [CENTRAL: CN-00730853] [PMID: ] - PubMed
-
- Van de Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. British Journal of Dermatology 2008;159(5):1177-85. [CENTRAL: CN-00681015] [PMID: ] - PubMed
VIP‐S trial 2020 {unpublished data only}
-
- NCT02690701. Study to evaluate the effect of secukinumab compared to placebo on aortic vascular inflammation in subjects with moderate to severe plaque psoriasis (VIP-S). clinicaltrials.gov/ct2/show/NCT02690701 (first received 24 February 2016).
VIP Trial 2018 {unpublished data only}
-
- Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circulation. Cardiovascular Imaging 2018;11(6):e007394. [CENTRAL: CN-01652064] [DOI: 10.1161/CIRCIMAGING.117.007394] - DOI - PMC - PubMed
-
- NCT01553058. Vascular Inflammation in psoriasis trial (the VIP trial) (VIP). clinicaltrials.gov/ct2/show/NCT01553058 (first received 14 February 2012). [CENTRAL: CN-01591340]
VIP‐U Trial 2020 {published data only}
-
- Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U Trial). Journal of Investigative Dermatology 2020;140(1):85-93.e2. - PMC - PubMed
-
- NCT02187172. Vascular inflammation in psoriasis-ustekinumab (VIP-U). clinicaltrials.gov/show/nct02187172 (first received 10 July 2014).
VOLTAIRE‐PSO 2021 {published data only}
-
- Menter A, Arenberger P, Balser S, Beissert S, Cauthen A, Czeloth N, et al. Similar efficacy, safety, and immunogenicity of the biosimilar BI 695501 and adalimumab reference product in patients with moderate-to-severe chronic plaque psoriasis: results from the randomized phase III VOLTAIRE-PSO study. Expert Opinion on Biological Therapy 2021;21(1):87-96. - PubMed
-
- Menter A, Arenberger P, Balser S, Beissert S. 13097 Biosimilar BI 695501 demonstrates clinical equivalence to adalimumab reference product in patients with moderate to severe chronic plaque psoriasis through 24 weeks. Journal of the American Academy of Dermatology 2020;83 (6 Suppl):AB5.
-
- NCT02850965. Efficacy, safety and immunogenicity of BI 695501 versus Humira® in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct02850965 (first received 1 August 2016). [CENTRAL: CN-01507166]
VOYAGE‐1 2016 {published data only}
-
- Armstrong AW, Reich K, Foley P, Han C, Song M, Shen YK, et al. Improvement in patient-reported outcomes (Dermatology Life Quality Index and the Psoriasis Symptoms and Signs Diary) with guselkumab in moderate-to-severe plaque psoriasis: results from the phase III VOYAGE 1 and VOYAGE 2 studies. American Journal of Clinical Dermatology 2018;20(1):155-64. [CENTRAL: CN-01943968] [PMID: ] - PMC - PubMed
-
- Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen Y-K, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. Journal of the American Academy of Dermatology 2016;76(3):405-17. [CENTRAL: CN-01341398] [PMID: ] - PubMed
-
- Griffiths CE, Papp KA, Kimball AB, Randazzo B, Wasfi Y, Li S, et al. Two-year efficacy and safety of guselkumab for treatment of moderate-to-severe psoriasis: phase 3 VOYAGE 1 trial. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1580-1. [CENTRAL: CN-01647499]
VOYAGE‐2 2017 {published data only}
-
- Reich K, Armstrong AW, Foley P, Song M, Miller M, Shen YK, et al. Maintenance of response through up to 4 years of continuous guselkumab treatment of psoriasis in the VOYAGE 2 phase 3 study. American Journal of Clinical Dermatology 2020;21(6):881-90. - PubMed
-
- Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. Journal of the American Academy of Dermatology 2017;76(3):418-31. [CENTRAL: CN-01341399] [PMID: ] - PubMed
Yang 2012 {published data only}
-
- Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX, Xu JH, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chinese Medical Journal 2012;125(11):1845-51. [CENTRAL: CN-00904898] [PMID: ] - PubMed
Ye 2020 {published data only}
-
- Ye T, Ye J, Zhang C. The effects of acitretin on patients with psoriasis vulgaris. International Journal of Clinical and Experimental Medicine 2020;13(7):5068-75.
Yilmaz 2002 {published data only}
-
- Yilmaz E, Yilmaz F, Yerebakan O. Re-PUVA therapy for psoriasis vulgaris: an effective choice. Journal of the European Academy of Dermatology and Venereology 2002;16(Suppl S1):258. [CENTRAL: CN-00416979]
-
- Yılmaz E, Yılmaz F, Yerebakan O. Re-PUVA therapy for psoriasis vulgaris: an effective choice [Psoriazis vulgaris tedavisinde etkili bir seçenek: asitretin ile PUVA kombinasyonu (Re-PUVA)]. Türkiye Klinikleri Dermatoloji Dergisi 2002;12(4):204-8. [CENTRAL: CN-01951242]
Yu 2019 {published data only}
-
- Yu Q, Tong Y, Cui L, Zhang L, Gong Y, Diao H, et al. Efficacy and safety of etanercept combined plus methotrexate and comparison of expression of pro-inflammatory factors expression for the treatment of moderate-to-severe plaque psoriasis. International Immunopharmacology 2019;73:442-50. - PubMed
Yu 2022 {published data only}
-
- Yu C, Zhang F, Ding Y, Li Y, Zhao Y, Gu J, et al. A randomized, double-blind phase III study to demonstrate the clinical similarity of biosimilar SCT630 to reference adalimumab in Chinese patients with moderate to severe plaque psoriasis. International Immunopharmacology 2022;112:109248. - PubMed
References to studies excluded from this review
Abe 2017 {published data only}
-
- Abe M, Nishigori C, Torii H, Ihn H, Ito K, Nagaoka M, et al. Tofacitinib for the treatment of moderate to severe chronic plaque psoriasis in Japanese patients: subgroup analyses from a randomized, placebo-controlled phase 3 trial. Journal of Dermatology 2017;44(11):1228-37. [CENTRAL: CN-01615689] - PMC - PubMed
Abufarag 2010 {published data only}
-
- Abufarag A, Aigner S, Czeloth N, Dalken B, Koch H, Niemann G, et al. Selective activation of naturally occurring regulatory T cells (Tregs) by the monoclonal antibody BT-061 as a novel therapeutic opportunity in psoriasis: early clinical results after single doses. Journal of Investigative Dermatology 2010;130(Suppl 2):S64. [CENTRAL: CN-00795758] [EMBASE: 70263767]
ACTRN12606000040561 {published data only}
-
- ACTRN12606000040561. The safety and efficacy of Cpn10 in psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12606000040561 (first received 27 January 2006).
Adsit 2017 {published data only}
-
- Adsit S, Zaldivar ER, Sofen H, Dei-Cas I, Garcia CM, Penaranda EO, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Advances in Therapy 2017;34(6):1327-39. [CENTRAL: CN-01443493] [DOI: 10.1007/s12325-017-0521-z] - DOI - PMC - PubMed
Akhyani 2010 {published data only}
-
- Akhyani M, Chams-Davatchi C, Hemami MR, Fateh S. Efficacy and safety of mycophenolate mofetil vs. methotrexate for the treatment of chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2010;24(12):1447-51. [CENTRAL: CN-00771805] [PMID: ] - PubMed
Al‐Oudah 2022 {published data only}
-
- Al-Oudah G A, Sahib A S, Al-Hattab M K, Al-Ameedee A A. Effect of CoQ10 administration to psoriatic Iraqi patients on biological therapy upon severity index (PASI) and quality of life index (DLQI) before and after therapy. Journal of Population Therapeutics & Clinical Pharmacology 2022;29(2):e52-60. - PubMed
Altmeyer 1994 {published data only}
-
- Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, et al. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. Journal of the American Academy of Dermatology 1994;30(6):977-81. [CENTRAL: CN-00101455] [PMID: ] - PubMed
Angsten 2007 {published data only}
-
- Angsten M, Schopf RE. Anti-TNF-alpha-therapy of psoriasis with infliximab or etanercept. Clinical, histological and immunohistochemical course. Aktuelle Dermatologie 2007;33(8-9):310-6. [CENTRAL: CN-00726884] [EMBASE: 2007503340]
Anonymous 2005 {published data only}
-
- Anonymous. Aldalimumab in psoriatic arthritis and as the initial therapy in rheumatoid arthritis [Adalimumab bei psoriasis-arthritis und zur initialtherapie bei rheumatoider arthritis]. Krankenpflege Journal 2005;43(7-10):244. [PMID: ] - PubMed
Anonymous 2008 {published data only}
-
- Anonymous. Trial watch: novel biologic for psoriasis shows superiority over current best-seller. Nature Reviews. Drug Discovery 2008;7(11):880-1. [PMID: ] - PubMed
Anonymous 2019 {published data only}
-
- Anonymous. Evaluation of effectiveness and safety in two optimization strategies with secukinumab in the treatment of moderate severe psoriasis. Journal of the American Academy of Dermatology 2019;81 (4 Suppl 1):AB60.
Araujo 2017 {published data only}
-
- Araujo EG, Englbrecht M, Hoepken S, Finzel S, Hueber A, Rech J, et al. Ustekinumab is superior to TNF inhibitor treatment in resolving enthesitis in PSA patients with active enthesitis-results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Annals of the Rheumatic Diseases 2017;76(Suppl 2):142. [CENTRAL: CN-01467990]
Araujo 2019 {published data only}
-
- Araujo EG, Englbrecht M, Hoepken S, Finzel S, Kampylafka E, Kleyer A, et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Seminars in Arthritis and Rheumatism 2019;48(4):632-7. [CENTRAL: CN-01930220] - PubMed
Arifov 1998 {published data only}
-
- Arifov S, Vaisov A, Ismagilov A, Abidova Z. Acitretin (neotigason) in the treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology 1998;11(Suppl 2):S290. [CENTRAL: CN-00465900]
Armati 1972 {published data only}
-
- Armati RP. Retinoic acid for psoriasis. Australasian Journal of Dermatology 1972;13(2):79-83. [CENTRAL: CN-00008047] [PMID: ] - PubMed
Asahina 2016 {published data only}
-
- Asahina A, Etoh T, Igarashi A, Imafuku S, Saeki H, Shibasaki Y, et al. Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double-blind, phase 3 study. Journal of Dermatology 2016;43(8):869-80. [CENTRAL: CN-01380021] [PMID: ] - PMC - PubMed
Augustin 2017 {published data only}
-
- Augustin M, Blome C, Paul C, Puig L, Luger T, Lambert J, et al. Quality of life and patient benefit following transition from methotrexate to ustekinumab in psoriasis. Journal of the European Academy of Dermatology and Venereology 2017;31(2):294-303. [CENTRAL: CN-01368710] [DOI: 10.1111/jdv.13823] - DOI - PubMed
Avgerinou 2011 {published data only}
-
- Avgerinou G, Tousoulis D, Siasos G, Oikonomou E, Maniatis K, Papageorgiou N, et al. Anti-tumor necrosis factor alpha treatment with adalimumab improves significantly endothelial function and decreases inflammatory process in patients with chronic psoriasis. International Journal of Cardiology 2011;151(3):382-3. [CENTRAL: CN-00860819] [PMID: ] - PubMed
Bachelez 2017 {published data only}
-
- Bachelez H, Griffiths CE, Papp K, Hall S, Merola JF, Feldman SR, et al. Effect of tofacitinib on efficacy and patient-reported outcomes in psoriasis patients with baseline psoriatic arthritis: a pooled analysis of 2 phase 3 studies. Arthritis and Rheumatology 2017;69(Suppl 10):613. [CENTRAL: CN-01602915]
Bagel 2017a {published data only}
-
- Bagel J, Duffin KC, Bukhalo M, Bobonich M, Gill A, Zhao F, et al. Ease of use and confidence using auto-injector to administer ixekizumab in a phase 3 trial evaluated with subcutaneous administration assessment questionnaire (SQAAQ). Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S14-5. [CENTRAL: CN-01713047]
Bagel 2017b {published data only}
-
- Bagel J, Tyring S, Rice KC, Collier DH, Kricorian G, Chung J, et al. Open-label study of etanercept treatment in patients with moderate-to-severe plaque psoriasis who lost a satisfactory response to adalimumab. British Journal of Dermatology 2017;177(2):411-8. [CENTRAL: CN-01393822] [DOI: 10.1111/bjd.15381] - DOI - PubMed
Bagel 2017c {published data only}
-
- Bagel J, Duffin KC, Moore A, Ferris LK, Siu K, Steadman J, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. Journal of the American Academy of Dermatology 2017;77(4):667-74. [CENTRAL: CN-01412958] - PubMed
Bagel 2018b {published data only}
-
- Bagel J, Samad AS, Stolshek BS, Aras GA, Chung JB, Kricorian G, et al. Open-label study to evaluate the efficacy of etanercept treatment in subjects with moderate to severe plaque psoriasis who have failed therapy with apremilast. Journal of Drugs in Dermatology 2018;17(10):1078-82. [CENTRAL: CN-01664523] - PubMed
Bagherani 2017 {published data only}
-
- Bagherani N, Smoller BR. Efficacy of topical tofacitinib, a Janus kinase inhibitor, in the treatment of plaque psoriasis. Dermatologic Therapy 2017;30(3):e12467. [CENTRAL: CN-01600736] [PMID: ] - PubMed
Bagot 1994 {published data only}
-
- Bagot M, Grossman R, Pamphile R, Binderup L, Charue D, Revuz J, et al. Additive effects of calcipotriol and cyclosporine A: from in vitro experiments to in vivo applications in the treatment of severe psoriasis. Comptes Rendus de l'Académie des Sciences. Série III, Sciences de la Vie 1994;317(3):282-6. [CENTRAL: CN-00107966] [PMID: ] - PubMed
Banerjee 2021 {published data only}
Bartlett 2008 {published data only}
-
- Bartlett BL, Tyring SK. Ustekinumab for chronic plaque psoriasis. Lancet 2008;371(9625):1639-40. [CENTRAL: CN-00631484] [PMID: ] - PubMed
Barzegari 2004 {published data only}
-
- Barzegari M, Ghaninejad H, Shizarpoor M. Comparison of bath PUVA and acitretin in treatment of psoriatic patients. Iranian Journal of Dermatology 2004;7(4):31-5. [CENTRAL: CN-00509588]
Batchelor 2009 {published data only}
-
- Batchelor JM, Ingram JR, Williams H. Adalimumab vs methotrexate for the treatment of chronic plaque psoriasis. Archives of Dermatology 2009;145(6):704-6. [CENTRAL: CN-01783607] [EMBASE: 2009300916] - PubMed
Bayerl 1992 {published data only}
-
- Bayerl C. Treatment of psoriasis vulgaris with etretinate versus cyclosporin A. Report on a study [Parallelgruppenvergleich etretinat versus cyclosporin A bei psoriasis vulgaris]. Aktuelle Dermatologie 1992;18(1-2):27-31. [CENTRAL: CN-00197510] [EMBASE: 1992096910]
Beissert 2009 {published data only}
-
- Beissert S, Pauser S, Sticherling M, Frieling U, Loske KD, Frosch PJ, et al. A comparison of mycophenolate mofetil with ciclosporine for the treatment of chronic plaque-type psoriasis. Dermatology (Basel, Switzerland) 2009;219(2):126-32. [CENTRAL: CN-00729115] [PMID: ] - PubMed
Berbis 1989 {published data only}
-
- Berbis P, Geiger JM, Vaisse C, Rognin C, Privat Y. Benefit of progressively increasing doses during the initial treatment with acitretin in psoriasis. Dermatologica 1989;178(2):88-92. [CENTRAL: CN-00058682] [PMID: ] - PubMed
Bhat 2017 {published data only}
-
- Bhat RM, Leelavathy B, Aradhya SS, Gopal MG, Pratap DV, Mubashir M, et al. Secukinumab efficacy and safety in Indian patients with moderate-to-severe plaque psoriasis: sub-analysis from FIXTURE, a randomized, placebo-controlled, phase 3 study. Indian Dermatology Online Journal 2017;8(1):16-24. [DOI: 10.4103/2229-5178.198765] - DOI - PMC - PubMed
Bhuiyan 2010 {published data only}
-
- Bhuiyan MS, Sikder MdA, Rashid MM, Rabin F. Role of oral colchicine in plaque type psoriasis. A randomized clinical trial comparing with oral methotrexate. Journal of Pakistan Association of Dermatologists 2010;20(3):146-51. [CENTRAL: CN-00789712] [EMBASE: 2010613015]
Bian 2018` {published data only}
-
- Bian M, Bissonnette R, Luger T, Thaçi D, Toth D, Xia S, et al. Secukinumab treatment in moderate-to-severe psoriasis patients demonstrates sustained low absolute PASI up to 4 years: results from SCULPTURE extension study. Australasian Journal of Dermatology 2018;59(Suppl 1):39. [CENTRAL: CN-01606960] [DOI: 10.1111/jdv.14878] - DOI
Bigby 2004 {published data only}
-
- Bigby M. A randomized controlled trial of methotrexate and cyclosporine in the treatment of psoriasis. Archives of Dermatology 2004;140(3):347-8. [CENTRAL: CN-00515754] [EMBASE: 2004120959] - PubMed
Bissonnette 2006 {published data only}
-
- Bissonnette R, Papp K, Poulin Y, Lauzon G, Aspeslet L, Huizinga R, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(3):472-8. [CENTRAL: CN-00555245] [PMID: ] - PubMed
Bissonnette 2010 {published data only}
-
- Bissonnette R, Papp K, Maari C, Yao Y, Robbie G, White WI, et al. A randomized, double-blind, placebo-controlled, phase I study of medi-545, an anti-interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. Journal of the American Academy of Dermatology 2010;62(3):427-36. [CENTRAL: CN-00734807] [PMID: ] - PubMed
Bissonnette 2015 {published data only}
-
- Bissonnette R, Iversen L, Sofen H, Griffiths CE, Foley P, Romiti R, et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. British Journal of Dermatology 2015;172(5):1395-406. [CENTRAL: CN-01254758] [PMID: ] - PubMed
Bissonnette 2017a {published data only}
-
- Bissonnette R, Luger T, Thaçi D, Toth D, Messina I, You R, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. British Journal of Dermatology 2017;177(4):1033-42. [CENTRAL: CN-01419893] [DOI: 10.1111/bjd.15706] - DOI - PubMed
Bissonnette 2017b {published data only}
-
- Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-α antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. Journal of Investigative Dermatology 2017;137(8):1638-45. [CENTRAL: CN-01410690] [DOI: 10.1016/j.jid.2017.02.977] - DOI - PubMed
Bissonnette 2018 {published data only}
-
- Bissonnette R, Haydey R, Rosoph LA, Lynde CW, Bukhalo M, Fowler JF, et al. Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: results from a double-blind, placebo-controlled, randomized study. Journal of the European Academy of Dermatology and Venereology 2018;32(3):403-10. [CENTRAL: CN-01643330] [DOI: 10.1111/jdv.14647] - DOI - PubMed
Bjerke 1989 {published data only}
-
- Bjerke JR, Geiger JM. Acitretin versus etretinate in severe psoriasis. A double-blind randomized Nordic multicenter study in 168 patients. Acta Dermato-Venereologica 1989;146(Suppl):206-7. [CENTRAL: CN-00064911] [PMID: ] - PubMed
Blauvelt 2016a {published data only}
-
- Blauvelt A, Papp K, Griffiths CE, Mallbris L, Dutronic Y, Ilo D, et al. Efficacy and safety of ixekizumab in patients previously treated with etanercept. Experimental Dermatology 2016;25(S4):38-9. [CENTRAL: CN-01407588] [DOI: 10.1111/exd.13200] - DOI
Blauvelt 2016b {published data only}
-
- Blauvelt A, Langley R, Leonardi C, Gordon K, Luger T, Ohtsuki M, et al. Ixekizumab, a novel anti-IL-17A antibody, exhibits low immunogenicity during long-term treatment in patients with psoriasis. Journal of Investigative Dermatology 2016;136(9 Suppl 2):S227. [CENTRAL: CN-01785592]
Blauvelt 2017a {published data only}
-
- Blauvelt A, Reich K, Lebwohl M, Burge D, Arendt C, Peterson L, et al. Certolizumab pegol for the treatment of patients with moderate-to-severe chronic plaque psoriasis: an overview of three randomized controlled trials. British Journal of Dermatology 2017;177(5):e249-50. [CENTRAL: CN-01452510] - PMC - PubMed
Blauvelt 2017b {published data only}
-
- Blauvelt A, Papp KA, Sofen H, Augustin M, Yosipovitch G, Katoh N, et al. Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. Journal of the European Academy of Dermatology and Venereology 2017;31(6):1004-13. [CENTRAL: CN-01459095] - PMC - PubMed
Blauvelt 2017c {published data only}
-
- Blauvelt A, Lacour JP, Fowler JF, Schuck E, Jauch-Lembach J, Balfour A, et al. Long-term efficacy, safety, and immunogenicity data from a phase III confirmatory study comparing GP2017, a proposed biosimilar, with reference adalimumab. American Journal of Gastroenterology 2017;112(Suppl 1):S419. [CENTRAL: CN-01463019]
Blauvelt 2017d {published data only}
-
- Blauvelt A, Reich K, Warren RB, Szepietowski JC, Sigurgeirsson B, Tyring SK, et al. Secukinumab re-initiation achieves regain of high response levels in patients who interrupt treatment for moderate to severe plaque psoriasis. British Journal of Dermatology 2017;177(3):879-81. [CENTRAL: CN-01622237] - PubMed
Blauvelt 2017e {published data only}
-
- Blauvelt A, Papp KA, Lebwohl MG, Green LJ, Hsu S, Bhatt V, et al. Rapid onset of action in patients with moderate-to-severe psoriasis treated with brodalumab: a pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). Journal of the American Academy of Dermatology 2017;77(2):372-4. [CENTRAL: CN-01622584] - PubMed
Blauvelt 2017f {published data only}
-
- Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, Leonardi CL, et al. Efficacy and safety of continuous ixekizumab treatment for 60 weeks in moderate-to-severe plaque psoriasis: results from the UNCOVER-3 trial. Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S15. [CENTRAL: CN-01713049]
Blauvelt 2017g {published data only}
-
- Blauvelt A, Papp KA, Griffiths CE, Puig L, Weisman J, Dutronc Y, et al. Efficacy and safety of switching to ixekizumab in etanercept non-responders: a subanalysis from two phase III randomized clinical trials in moderate-to-severe plaque psoriasis (UNCOVER-2 and -3). American Journal of Clinical Dermatology 2017;18(2):273-80 Correction in American Journal of Clinical Dermatology 2018; 19(3):457. [CENTRAL: CN-01368347] - PMC - PubMed
Blauvelt 2017h {published data only}
-
- Blauvelt A, Reich K, Warren R, Sigurgeirsson B, Langley R, Papavassilis C, et al. Secukinumab retreatment shows rapid recapture of treatment response: an analysis of a phase 3 extension trial in psoriasis. International Journal of Dermatology 2017;56(11):1264-5. [CENTRAL: CN-01570754]
Blauvelt 2017i {published data only}
-
- Blauvelt A, Ferris LK, Yamauchi PS, Qureshi A, Leonardi CL, Farahi K, et al. Extension of ustekinumab maintenance dosing interval in moderate-to-severe psoriasis: results of a phase IIIb, randomized, double-blinded, active-controlled, multicentre study (PSTELLAR). British Journal of Dermatology 2017;177(6):1552-61. [CENTRAL: CN-01428390] - PubMed
Blauvelt 2017j {published data only}
-
- Blauvelt A, Lebwohl MG, Green LJ, Hsu S, Bhatt V, Rastogi S, et al. Median time to treatment response in patients with moderate-to-severe plaque psoriasis treated with brodalumab 210mg or ustekinumab: a pooled analysis of data from two phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). Journal of Clinical and Aesthetic Dermatology 2017;10(5 Suppl 1):S22-3. [CENTRAL: CN-01628810] - PubMed
Blauvelt 2017k {published data only}
-
- Blauvelt A, Gooderham M, Iversen L, Ball S, Zhang L, Agada N, et al. Efficacy and safety of ixekizumab for the treatment of plaque psoriasis: results through 108 weeks randomised, phase III clinical trial (UNCOVER-3). Journal of Investigative Dermatology 2017;137(10 Suppl 2):S260. [CENTRAL: CN-01416619] - PubMed
Blauvelt 2018a {published data only}
-
- Blauvelt A, Reich K, Papp KA, Tyring SK, Sinclair R, Thaçi D, et al. Predictors of response to tildrakizumab for moderate to severe chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):22. [CENTRAL: CN-01620162]
Blauvelt 2018b {published data only}
-
- Blauvelt A, Sofen H, Papp K, Gooderham M, Zhao Y, Lowry S, et al. Tildrakizumab efficacy over time by week 28 response levels in two phase 3 clinical trials in patients with chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):49. [CENTRAL: CN-01920779]
Blauvelt 2018c {published data only}
-
- Blauvelt A, Tyring S, Philipp S, Adam D, Song M, Wasf Y, et al. Speed of response of guselkumab compared with adalimumab for the treatment of moderate-to-severe psoriasis: results through week 24 from the phase 3, double-blinded, placebo-and active comparator-controlled voyage 1 and voyage 2 trials. Acta Dermato-Venereologica 2018;98(Suppl 219):21. [CENTRAL: CN-01620164]
Blauvelt 2018d {published data only}
-
- Blauvelt A, Strober B, Langley R, Burge D, Pisenti L, Yassine M, et al. Safety of certolizumab pegol over 48 weeks in chronic plaque psoriasis phase 3 trials. Acta Dermato-Venereologica 2018;98(Suppl 219):21. [CENTRAL: CN-01620163]
Blauvelt 2018e {published data only}
-
- Blauvelt A, Reich K, Lebwohl M, Burge D, Arendt C, Peterson L, et al. Certolizumab pegol for the treatment of patients with moderate-to-severe chronic plaque psoriasis: pooled analysis of week 16 data from three randomized controlled trials. Journal of the European Academy of Dermatology and Venereology 2018;33(3):546-52. [CENTRAL: CN-01915790] - PMC - PubMed
Blauvelt 2018f {published data only}
-
- Blauvelt A, Muram TM, See K, Mallinckrodt CH, Crowley JJ, Van de Kerkhof P. Improvements in psoriasis within different body regions vary over time following treatment with ixekizumab. Journal of Dermatological Treatment 2018;29(3):220-9. [CENTRAL: CN-01604313] - PubMed
Blauvelt 2018g {published data only}
-
- Blauvelt A, Reich K, Papp KA, Kimball AB, Gooderham M, Tyring SK, et al. Safety of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomized controlled trials. British Journal of Dermatology 2018;179(3):615-22. [CENTRAL: CN-01645090] - PubMed
Blauvelt 2018h {published data only}
-
- Blauvelt A, Tyring S, Gooderham M, Koo J, Zhao Y, Lowry S, et al. Better skin clearance is associated with improved quality of life in moderate-to-severe psoriasis patients treated with tildrakizumab. Acta Dermato-Venereologica 2018;98(Suppl 219):31-2. [CENTRAL: CN-01620179]
Blauvelt 2020 {published data only}
-
- Blauvelt A, Merola JF, Papp KA, Gooderham M, Gottlieb AB, Cioffi C, et al. 14020 Durability of responses with bimekizumab, a selective dual inhibitor of interleukin-17A and -17F, in moderate to severe chronic plaque psoriasis in a 60-week randomized, double-blinded, phase 2b study (BE ABLE 2). Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB16.
-
- Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, et al. Bimekizumab for patients with moderate-to-severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled phase 2b extension study. Journal of the American Academy of Dermatology 2020;83(5):1367-74. - PubMed
Branigan 2017 {published data only}
-
- Branigan P, Liu X, Chen Y, Scott B, Yao Z, Li S, et al. Sustained response following withdrawal of guselkumab treatment correlates with reduced Th17 and Th22 effector cytokine levels. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S194. [CENTRAL: CN-01416625]
Brasil 2012 {published data only}
-
- Brasil, Ministério da Saúde, Departamento de Gestão e Incorporação de Tecnologias em, Saúde. Medicamentos biológicos (infliximabe, etanercepte, adalimumabe e ustequinumabe) para o tratamento da psoríase moderada a grave em adultos. conitec.gov.br/images/Incorporados/Biologicos-Psoriase-final.pdf 2012 (accessed prior to 17 December 2019).
Brasil 2013 {published data only}
-
- Brasil, Ministério da Saúde, Departamento de Gestão e Incorporação de Tecnologias em, Saúde. Golimumabe para artrite psoriásica. conitec.gov.br/images/Incorporados/Golimumabe-ArtritePsoriasica-final.pdf 2013 (accessed prior to 17 December 2019).
Brasil 2016 {published data only}
-
- Brasil, Ministério da Saúde, Comissão Nacional de Incorporação de Tecnologias no SUS. Golimumabe para o tratamento da artrite psoriásica. conitec.gov.br/images/Relatorios/2016/Relatorio_Golimumabe_ArtritePsoria... 2016 (accessed prior to 17 December 2019).
Buono 2020 {published data only}
-
- Buono R, Parisi A, Russo R, Mastrobuoni C, Di Monda G, Valentino U, et al. Efficacy and tolerability about ustekinumab in patients withpsoriatic arthritis after 24 months of treatment. Italian Journal of Medicine 2020;14 (Suppl 2):18.
Burden 2017 {published data only}
-
- Burden AD. Etanercept or infliximab for psoriasis? An independent randomized clinical trial. British Journal of Dermatology 2017;176(3):565. [CENTRAL: CN-01336581] - PubMed
Burkhardt 2017 {published data only}
-
- Burkhardt N, Mrowietz U, Carrascosa JM, Fernandez-Penas P, Guede D, Wilhelm S, et al. Absolute and relative PASI over 1 year of treatment with ixekizumab (IXE): descriptive analysis in patients with moderate-to-severe plaque psoriasis. Australasian Journal of Dermatology 2017;58(Suppl 1):42. [CENTRAL: CN-01378807]
Callis Duffin 2017 {published data only}
-
- Callis Duffin K, Bagel J, Bukhalo M, Mercado Clement IJ, Choi SL, Zhao F, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). Journal of the European Academy of Dermatology and Venereology 2017;31(1):107-13. [CENTRAL: CN-01368719] [PMID: ] - PMC - PubMed
Cassano 2006 {published data only}
-
- Cassano N, Loconsole F, Galluccio A, Miracapillo A, Pezza M, Vena GA. Once-weekly administration of high-dosage etanercept in patients with plaque psoriasis: results of a pilot experience (power study). International Journal of Immunopathology and Pharmacology 2006;19(1):225-9. [CENTRAL: CN-00563725] - PubMed
Cassano 2010 {published data only}
-
- Cassano N, Loconsole F, Miracapillo A, Travaglini M, Digiuseppe MD, Congedo M, et al. Treatment of psoriasis with different dosage regimens of etanercept: preliminary results from the Talpharanta Plastic Study Group. International Journal of Immunopathology and Pharmacology 2010;23(3):797-802. [PMID: ] - PubMed
Cather 2006 {published data only}
-
- Cather J, Krueger G, Jackson M, Samstov A. Efficacy and safety of low-dose acitretin for the treatment of moderate to severe plaque-type psoriasis. Journal of the American Academy of Dermatology (American Academy of Dermatology 64th Annual Meeting March 3-7, 2006) 2006;54(3 Suppl):AB217 (abstract P2877). [CENTRAL: CN-00602420]
Cather 2018 {published data only}
-
- Cather JC, Meeuwis K, Burge R, Bleakman AP, Lin CY, Gottlieb A, et al. Ixekizumab improves impact of genital psoriasis on sexual activity: results from a phase 3b study. Acta Dermato-Venereologica 2018;98(Suppl 219):11. [CENTRAL: CN-01620184]
Chakravadhanula 2017 {published data only}
-
- Chakravadhanula U, Chandrashekar BS, Parekh M, Krupashankar DS, Swaroop HS, Pawar D. One-year pilot study to evaluate sequential therapy with ciclosporin and itolizumab in treatment of chronic plaque psoriasis. British Journal of Dermatology 2017;177(5):e291. [CENTRAL: CN-01452514]
Chapman 2018 {published data only}
-
- Chapman M, Cirulli J, McBride S. Sustained improvement in patient-reported outcomes with continued apremilast treatment over 104 weeks in patients with moderate to severe psoriasis. Australasian Journal of Dermatology 2018;59(Suppl 1):46. [CENTRAL: CN-01606954]
ChiCTR2000030273 2020 {published data only}
-
- ChiCTR2000030273. A comparative study to evaluate the pharmacokinetics and safety of BAT2206 injection vs ustekinumab injection (Stelara) in healthy Chinese male subjects. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000030273 (first received 27 February 2020).
ChiCTR‐INR‐16009710 {unpublished data only}
-
- ChiCTR-INR-16009710. Acitretin plus methotrexate in the treatment of moderate to severe psoriasis vulgaris [The role of keratin 17 in the pathogenesis of psoriasis through PI3K/Akt signal pathways]. www.chictr.org.cn/showprojen.aspx?proj=16444/ChiCTR-INR-16009710 (first received 2 November 2016).
Chládek 2002 {published data only}
Chodorowska 1999a {published data only}
-
- Chodorowska G, Czelej D, Juszkiewicz-Borowiec M, Pietrzak A, Wojnowska D, Krasowska D. Selected cytokines and acute phase proteins in psoriatic patients treated with cyclosporin A or Re-PUVA methods. Annales Universitatis Mariae Curie-Sklodowska. Section D: Medicina 1999;54:173-80. [CENTRAL: CN-00325819] - PubMed
Chodorowska 1999b {published data only}
-
- Chodorowska G, Czelej D, Juszkiewicz-Borowiec M, Pietrzak A, Wojnowska D, Krasowska D. Plasma levels of selected cytokines and acute phase proteins in 2 groups of psoriatic patients treated with cyclosporine A or RE-PUVA method. Journal of the European Academy of Dermatology and Venereology 1999;12(Suppl 2):S330. [CENTRAL: CN-00478492] - PubMed
Choi 2017 {published data only}
-
- Choi CW, Kim BR, Seo E, Youn SW. The objective Psoriasis Area and Severity Index: a randomized controlled pilot study comparing the effectiveness of ciclosporin and methotrexate. British Journal of Dermatology 2017;177(6):1740-1. [CENTRAL: CN-01914246] - PubMed
Crowley 2018a {published data only}
-
- Crowley J, Gisondi P, Geng Z, Servin OR. Long-term safety and efficacy of adalimumab from the phase 3 randomized, placebo-controlled trial in patients with nail and skin psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):25. [CENTRAL: CN-01620198]
Crowley 2018b {published data only}
-
- Crowley J, Papp KA, Hong C, Parno J, Mendelsohn AM, Li Q, et al. Efficacy of tildrakizumab in etanercept partial responders or nonresponders. Acta Dermato-Venereologica 2018;98(Suppl 219):29. [CENTRAL: CN-01620188]
CTRI/2018/01/011373 {published data only}
-
- CTRI/2018/01/011373. Comparison of efficacy and safety of oral vs subcutaneous methotrexate in psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/01/011373 (first received 16 January 2018).
CTRI/2020/07/026598 2020 {published data only}
-
- CTRI/2020/07/026598. A comparative study for the efficacy and side effects in subcutaneous and oral methotrexate in patients of chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/07/026598 (first received 15 July 2020).
CTRI/2020/12/029472 2020 {published data only}
-
- CTRI/2020/12/029472. Fasting in managment of psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/029472 (first received 1 December 2020).
CTRI/2020/12/029611 {published data only}
-
- CTRI/2020/12/029611. A study to check effectiveness of dimethyl fumarate gastro-resistant hard capsule in patients with moderate to severe chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/029611 (first received 7 December 2020).
De Jong 2003 {published data only}
-
- De Jong EM, Mork NJ, Seijger MM, De La Brassine M, Lauharanta J, Jansen CT, et al. The combination of calcipotriol and methotrexate compared with methotrexate and vehicle in psoriasis: results of a multicentre placebo-controlled randomized trial. British Journal of Dermatology 2003;148(2):318-25. [CENTRAL: CN-00422725] - PubMed
De Mendizabal 2017 {published data only}
-
- De Mendizabal NV, Heathman M, Jackson K. A longitudinal PKPD model describing the effect of ixekizumab on static physician's global assessment score (sPGA) in patients with moderate-to-severe plaque psoriasis. Journal of Pharmacokinetics and Pharmacodynamics 2017;44(1 Suppl 1):S102.
Dubiel 1972 {published data only}
-
- Dubiel W, Happle R. Experimental treatment with fumaric acid monoethylester in psoriasis vulgaris [Behandlungsversuch mit fumarsauremonoathylester bei psoriasis vulgaris]. Zeitschrift fur Haut- und Geschlechtskrankheiten 1972;47(13):545-50. [PMID: ] - PubMed
Duffin 2016 {published data only}
-
- Duffin KC, Bagel J, Bukhalo M, Clement IJ, Zhao F, Gill A, et al. Comparison of the pharmacokinetics of ixekizumab following subcutaneous administration using a prefilled syringe versus an autoinjector in patients with moderate-to-severe psoriasis. Journal of the American Academy of Dermatology 2016;74(5 Suppl 1):AB242. [EMBASE: 72275926] - PMC - PubMed
Duffin 2017 {published data only}
-
- Duffin KC, Papp KA, Bagel J, Pharm DEL, Chen R, Gottlieb AB. Evaluation of the physician global assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. Journal of Drugs in Dermatology 2017;16(2):147-53. [CENTRAL: CN-01416699] - PubMed
Ecker‐Schlipf 2009 {published data only}
-
- Ecker-Schlipf B. Psoriasis vulgaris: How effective and safe is the calcineurin inhibitor voclosporin? [Psoriasis vulgaris: Wie wirksam und sicher ist der calcineurin- inhibitor voclosporin?]. Arzneimitteltherapie 2009;27(3):97-8. [EMBASE: 2009143373]
Edson‐Heredia 2013 {published data only}
-
- Edson-Heredia E, Banerjee S, Zhu B, Maeda-Chubachi T, Cameron G, Shen W, et al. A PASI ≥ 90 response is associated with improved patient reported outcomes: results from a phase 2 study in patients with psoriasis treated with ixekizumab. Journal of the European Academy of Dermatology and Venereology 2013;27(Suppl 4):25. [DOI: 10.1111/jdv.12186] - DOI - PubMed
Egeberg 2016 {published data only}
Elewski 2007 {published data only}
-
- Elewski B, Leonardi C, Gottlieb AB, Strober BE, Simiens MA, Dunn M, et al. Comparison of clinical and pharmacokinetic profiles of etanercept 25 mg twice weekly and 50 mg once weekly in patients with psoriasis. British Journal of Dermatology 2007;156(1):138-42. [CENTRAL: CN-00577519] - PubMed
Elewski 2017 {published data only}
-
- Elewski BE, Puig L, Mordin M, Gilloteau I, Sherif B, Fox T, et al. Psoriasis patients with Psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75-89 response: results from two phase 3 studies of secukinumab. Journal of Dermatological Treatment 2017;28(6):492-9. [CENTRAL: CN-01443627] - PubMed
Elewski 2018a {published data only}
-
- Elewski B, Menter M, Crowley J, Tyring J, Zhao Y, Lowry S, et al. Sustained and improved efficacy of tildrakizumab from week 28 to week 52 in treating moderate-to-severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S25. [CENTRAL: CN-01713707]
Elewski 2018b {published data only}
-
- Elewski B, Menter A, Crowley J, Tyring S, Zhao Y, Lowry S, et al. Sustained and improved efficacy of tildrakizumab from week 28 to week 52 in treating moderate-to-severe plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):31. [CENTRAL: CN-01620180]
Ellis 1986 {published data only}
-
- Ellis CN, Gorsulowsky DC, Hamilton TA, Billings JK, Brown MD, Headington JT, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA 1986;256(22):3110-6. [CENTRAL: CN-00045553] - PubMed
Ellis 2001 {published data only}
-
- Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. New England Journal of Medicine 2001;345(4):248-55. [CENTRAL: CN-00349381] [PMID: ] - PubMed
-
- Ellis CN, Mordin MM, Adler EY. Effects of alefacept on health-related quality of life in patients with psoriasis: results from a randomized, placebo-controlled phase II trial. American Journal of Clinical Dermatology 2003;4(2):131-9. [CENTRAL: CN-00435105] [PMID: ] - PubMed
Ellis 2002 {published data only}
-
- Ellis CN, Reiter KL, Bandekar RR, Fendrick AM. Cost-effectiveness comparison of therapy for psoriasis with a methotrexate-based regimen versus a rotation regimen of modified cyclosporine and methotrexate. Journal of the American Academy of Dermatology 2002;46(2):242-50. [CENTRAL: CN-01783592] [PMID: ] - PubMed
Ellis 2012 {published data only}
-
- Chow C, Zhang Z, Goldfarb MT, Simpson MJ, Ellis CN. Evaluation of Psoriasis Area and Severity Index, Static Physician's Global Assessment, and Lattice System - Physician's Global Assessment for assessing severity of psoriasis. Journal of the American Academy of Dermatology 2012;131(Suppl 1):S81. [CENTRAL: CN-00843715]
Engst 1989 {published data only}
-
- Engst R, Huber J. Results of cyclosporin treatment of severe, chronical psoriasis vulgaris. Hautarzt 1989;40(8):486-9. [EMBASE: 1989202264] - PubMed
Erkko 1997 {published data only}
-
- Erkko P, Granlund H, Nuutinen M, Reitamo S. Comparison of cyclosporin A pharmacokinetics of a new microemulsion formulation and standard oral preparation in patients with psoriasis. British Journal of Dermatology 1997;136(1):82-8. [PMID: ] - PubMed
EUCTR2007‐004328‐18‐FR {published data only}
-
- EUCTR2007-004328-18-FR. A 12 week double-blind, randomised, placebo-controlled, modified dose-escalation trial to investigate safety, efficacy, and pharmacokinetics of BIRT 2584XX tablets at doses of 100, 300 and 500 mg administered once daily in patients with moderate to severe psoriasis with a 12 week treatment extension for PASI 50 responders. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-004328-18-FR (first received 14 November 2007).
EUCTR2012‐005685‐35‐DE {published data only}
-
- EUCTR2012-005685-35-DE. A study to compare the efficacy of a new developed product, FP187, to a marketed product, to each other but also to placebo, in patients with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-005685-35-DE (first received 26 July 2013).
EUCTR2016‐001593‐15‐ES {published data only}
-
- EUCTR2016-001593-15-ES. Study to evaluate the efficacy and safety of high induction doses of adalimumab in moderate to severe psoriasis patients. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-001593-15-ES (first received 23 August 2016).
EUCTR2016‐003592‐21‐GB {published data only}
-
- EUCTR2016-003592-21-GB. Evaluating the benefits of using secukinumab rather than standard treatments as the first systemic treatment in moderate to severe psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-003592-21-GB (first received 17 October 2016).
EUCTR2018‐001021‐10‐SE {published data only}
-
- EUCTR2018-001021-10-SE. A clinical study with brodalumab for patients suffering from psoriasis and not benefitting the TNF-alpha treatment. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001021-10-SE (first received 17 May 2018).
EUCTR2019‐000817‐35‐DE {published data only}
-
- EUCTR2019-000817-35-DE. An open-label, randomized, phase IV study, to assess the efficacy and safety of tildrakizumab in patients with moderate to severe chronic plaque psoriasis who are non-responders to dimethyl fumarate therapy. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-000817-35-DE (first received 6 June 2019).
-
- NCT04263610. Efficacy and safety of tildrakizumab in participants with moderate-to-severe chronic plaque psoriasis who are non-responders to dimethyl fumarate therapy (TRANSITION). clinicaltrials.gov/show/NCT04263610 (first received 11 February 2020).
EUCTR2021‐000542‐18‐LV {published data only}
-
- EUCTR2021-000542-18-LV. A study to investigate the impact of switching between ABP 501 and adalimumab for the treatment of subjects with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-000542-18-LV (first received 22 October 2021).
EUCTR2022‐000695‐19‐LT {published data only}
-
- EUCTR2022-000695-19-LT. Clinical study assessing interchangeability between SB5 and Humira in patients with moderate to severe chronic plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2022-000695-19-LT (first received 2 May 2022).
EXCEED 2021 {published data only}
-
- Gottlieb AB, Merola JF, Reich K, Behrens F, Nash P, Griffiths CEM, et al. Efficacy of secukinumab and adalimumab in psoriatic arthritis patients with concomitant moderate to severe plaque psoriasis: results from the EXCEED, a randomised, double-blind head-to-head monotherapy study. British Journal of Dermatology 2021 Apr 29 [Epub ahead of print]. [DOI: 10.1111/bjd.20413] - DOI - PMC - PubMed
Ezquerra 2007 {published data only}
-
- Ezquerra GM, Regana MS, Millet PU. Combination of acitretin and oral calcitriol for treatment of plaque-type psoriasis. Acta Dermato-Venereologica 2007;87(5):449-50. [CENTRAL: CN-00619032] - PubMed
Feldman 2017 {published data only}
-
- Feldman SR, Green L, Kimball AB, Siu K, Zhao Y, Herrera V, et al. Secukinumab improves scalp pain, itching, scaling and quality of life in patients with moderate-to-severe scalp psoriasis. Journal of Dermatological Treatment 2017;28(8):716-21. [CENTRAL: CN-01454207] - PubMed
Fernandes 2013 {published data only}
-
- Fernandes IC, Torres T, Selores M. Maintenance treatment of psoriasis with cyclosporine A: comparison between continuous and weekend therapy. Journal of the American Academy of Dermatology 2013;68(2):341-2. [CENTRAL: CN-00841435] - PubMed
Fernandez 2017 {published data only}
-
- Fernandez Penas P, Goldblum O, Berggren L, Burkhardt N, Jullien D. Long-term clinical outcomes after 2 years of ixekizumab treatment in patients with moderate-to-severe psoriasis with a focus on absolute PASI. Journal of Investigative Dermatology 2017;137(5 Suppl 1):S58. [CENTRAL: CN-01375458]
Finzi 1993 {published data only}
-
- Italian Multicenter Study Group on Cyclosporin in Psoriasis. Cyclosporin versus etretinate: Italian multicenter comparative trial in severe plaque-form psoriasis. Dermatology (Basel, Switzerland) 1993;187(Suppl 1):8-18. [CENTRAL: CN-00095652] - PubMed
Fitz 2018 {published data only}
-
- Fitz L, Zhang W, Soderstrom C, Fraser S, Lee J, Quazi A, et al. Association between serum interleukin-17A and clinical response to tofacitinib and etanercept in moderate to severe psoriasis. Clinical and Experimental Dermatology 2018;43(7):790-7. [CENTRAL: CN-01923580] - PubMed
Fleischer 2005 {published data only}
-
- Fleischer AB, Carroll C, Hartle JE, Krejci-Manwaring J, McCarty MA, Feldman SR. A randomized, double-blind, right/left comparative study of the efficacy of acitretin with and without the co-administration of 0.1 percent tacrolimus ointment in the treatment of moderate to severe psoriasis. Journal of Investigative Dermatology 2005;124(4 Suppl):A46. [CENTRAL: CN-00550944]
Foley 2017 {published data only}
-
- Foley P, Song M, Shen YK, You Y, Wasfi Y, Griffiths CE. Guselkumab treatment provided higher frequency of complete skin clearance compared with adalimumab treatment among patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2017;177(5):e273-4. [DOI: 10.1111/bjd.16059] - DOI
Foley 2018 {published data only}
Fredriksson 1971 {published data only}
-
- Fredriksson T. Antipsoriatic activity of retinoic acid (vitamin A acid). Dermatologica 1971;142(3):133-6. [CENTRAL: CN-00006362] - PubMed
Fredriksson 1978 {published data only}
-
- Fredriksson T, Pettersson U. Severe psoriasis - oral therapy with a new retinoid. Dermatologica 1978;157(4):238-44. [CENTRAL: CN-00382865] - PubMed
Friedrich 2001 {published data only}
-
- Friedrich M, Sterry W, Klein A, Ruckert R, Docke WD, Asadullah K. Addition of pentoxifylline could reduce the side effects of fumaric acid esters in the treatment of psoriasis. Acta Dermato-Venereologica 2001;81(6):429-30. [CENTRAL: CN-00388555] - PubMed
GAIN 2021 {published data only}
-
- Reich K, Korber A, Mrowietz U, Sticherling M, Sieder C, Fruh J, et al. Secukinumab 2-weekly vs. 4-weekly dosing in patients with plaque-type psoriasis: results from the randomized GAIN study. British Journal of Dermatology 2021;184(5):849-56. - PubMed
Gambichler 2011 {published data only}
-
- Gambichler T, Tigges C, Scola N, Weber J, Skrygan M, Bechara FG, et al. Etanercept plus narrowband ultraviolet b phototherapy of psoriasis is more effective than etanercept monotherapy at 6 weeks. British Journal of Dermatology 2011;164(6):1383-6. [CENTRAL: CN-00812147] - PubMed
Ganguly 2004 {published data only}
-
- Ganguly R, Singh A, Sato R. Etanercept therapy provides clinically meaningful improvement in dermatology quality of life index in patients with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2004;18(6):807. [CENTRAL: CN-00550919]
Gil 2003 {published data only}
-
- Gil JM, Sanchez-Regana M, PaIazon DB, Cuchillero RO, Ezquerra GM, Millet PU. Association between calcitriol per os and acitretinoin in the treatment of psoriasis. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):383. [CENTRAL: CN-00478546]
Gisondi 2020 {published data only}
-
- Gisondi P, Bellinato F, Bruni M, De Angelis G, Girolomoni G. Methotrexate vs secukinumab safety in psoriasis patients with metabolic syndrome. Dermatologic Therapy 2020;33(6):e14281. - PubMed
Glatt 2017 {published data only}
-
- Glatt S, Helmer E, Haier B, Strimenopoulou F, Price G, Vajjah P, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. British Journal of Clinical Pharmacology 2017;83(5):991-1001. [CENTRAL: CN-01447402] - PMC - PubMed
Goerz 1978 {published data only}
-
- Goerz G, Orfanos CE. Systemic treatment of psoriasis with a new aromatic retinoid. Preliminary evaluation of a multicenter controlled study in the Federal Republic of Germany. Dermatologica 1978;157(Suppl 1):38-44. [PMID: ] - PubMed
Gold 2018 {published data only}
-
- Gold LS, Forman S, Lebwohl M, Jackson JM, Goncalves J, Levi E, et al. Impact on quality of life and satisfaction with apremilast in patients with moderate plaque psoriasis: 52-week results of the UNVEIL study. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S23-4. [CENTRAL: CN-01713690]
Goll 2017 {published data only}
-
- Goll GL, Jorgensen KK, Sexton J, Olsen IC, Bolstad N, Lorentzen M, et al. Long-term safety and efficacy of biosimilar infliximab (CT-P13) after switching from originator infliximab: results from the 26-week open label extension of a randomized Norwegian trial. Arthritis and Rheumatology 2017;69(Suppl 10):2800. [CENTRAL: CN-01423748]
Goll 2018 {published data only}
-
- Goll GL, Jorgensen KK, Sexton J, Olsen IC, Bolstad N, Lorentzen M, et al. Long-term safety and efficacy of biosimilar infliximab (CT-P13) after switching from originator infliximab: results from the 26-week open label extension of a Norwegian randomised trial. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1383-4. [CENTRAL: CN-01647461] [DOI: 10.1136/annrheumdis-2018-eular.4620] - DOI
Gollnick 1988 {published data only}
-
- Gollnick H, Bauer R, Brindley C, Orfanos CE, Plewig G, Wokalek H, et al. Acitretin versus etretinate in psoriasis. Clinical and pharmacokinetic results of a German multicenter study. Journal of the American Academy of Dermatology 1988;19(3):458-68. [CENTRAL: CN-00055942] - PubMed
Gollnick 1993 {published data only}
-
- Gollnick HP, Zaun H, Ruzicka T, Sommerburg C, Loew S, Mahrle G, et al. Relapse rate of severe generalized psoriasis after treatment with acitretin or etretinate. Results of the first randomized double-blind multicenter half-year follow-up study. European Journal of Dermatology 1993;3(6):442-6. [CENTRAL: CN-00181049]
Gollnick 2002 {published data only}
-
- Gollnick H, Altmeyer P, Kaufmann R, Ring J, Christophers E, Pavel S, et al. Topical calcipotriol plus oral fumaric acid is more effective and faster acting than oral fumaric acid monotherapy in the treatment of severe chronic plaque psoriasis vulgaris. Dermatology (Basel, Switzerland) 2002;205(1):46-53. [CENTRAL: CN-00397743] - PubMed
Gordon 2014 {published data only}
-
- Gordon K, Leonardi C, Braun D, Cameron G, Erickson J, Lebwohl M, et al. Results after at least 52 weeks of open label treatment with ixekizumab, an anti-IL-17A monoclonal antibody, in a phase 2 study in chronic plaque psoriasis. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB183. [CENTRAL: CN-01057458]
Gordon 2015 {published data only}
-
- Gordon KB, Leonardi C, Lebwohl M, Cameron G, Erickson J, Braun D, et al. Results after at least 52 weeks of open label treatment with ixekizumab, an anti-IL-17A monoclonal antibody, in a phase 2 study in chronic plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2015;8(5 Suppl 1):S17-8. [CENTRAL: CN-01378778]
Gordon 2018a {published data only}
-
- Gordon K, Armstrong A, Foley P, Wasfi Y, Song M, Shen YK, et al. Long-term efficacy of guselkumab treatment after drug withdrawal and retreatment in patients with moderate-severe plaque psoriasis: results from voyage 2. Acta Dermato-Venereologica 2018;98(Suppl 219):22-3. [CENTRAL: CN-01620161] [DOI: 10.2340/00015555-2978] - DOI
Gordon 2018b {published data only}
-
- Gordon K, Reich K, Pariser D, Menter A, Tyring S, Sofen H, et al. Efficacy of tildrakizumab in moderate to severe psoriasis patients with prior exposure to apremilast. Acta Dermato-Venereologica 2018;98(Suppl 219):29-30. [CENTRAL: CN-01620187] [DOI: 10.2340/00015555-2978] - DOI
Gordon 2018c {published data only}
-
- Gordon K, Crowley J, Poulin Y, Mendelsohn A, Parno J, Rozzo S, et al. Disease severity and efficacy insights: patient-level pasi scores in tildrakizumab psoriasis trials. Acta Dermato-Venereologica 2018;98(Suppl 219):30-1. [DOI: 10.2340/00015555-2978] - DOI
Gordon 2018d {published data only}
-
- Gordon KB, Armstrong AW, Han C, Foley P, Song M, Wasfi Y, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the phase 3 VOYAGE 2 study. Journal of the European Academy of Dermatology and Venereology 2018;32(11):1940-9. [CENTRAL: CN-01617890] - PubMed
Gottlieb 2002 {published data only}
-
- Gottlieb AB, Vaishnaw K, Rizova E. Alefacept (AMEVIVETM) does not blunt primary or secondary immune responses. Journal of Investigative Dermatology 2002;118(6):1098. [CENTRAL: CN-00790440]
Gottlieb 2003b {published data only}
-
- Gottlieb AB, Casale TB, Frankel E, Goffe B, Lowe N, Ochs HD, et al. CD4+ T-cell-directed antibody responses are maintained in patients with psoriasis receiving alefacept: results of a randomized study. Journal of the American Academy of Dermatology 2003;49(5):816-25. [CENTRAL: CN-00474727] - PubMed
Gottlieb 2003c {published data only}
-
- Gottlieb AB, Feng A, Zitnik R. Prolonged response durability following ENBRELA® (etanercept) monotherapy. Journal of Investigative Dermatology 2003;121(1):68. [CENTRAL: CN-00795277]
Gottlieb 2004b {published data only}
-
- Gottlieb B, Goffe B, Veith J, Stevens S, Nakanishi A. Safety of etanercept in an integrated multistudy database of patients with psoriasis. Journal of Investigative Dermatology 2004;122(3):A55. [CENTRAL: CN-00509648]
Gottlieb 2005 {published data only}
-
- Gottlieb AB, Griffiths CE, Ho VC, Lahfa M, Mrowietz U, Murrell DF, et al. Oral pimecrolimus in the treatment of moderate to severe chronic plaque-type psoriasis: a double-blind, multicentre, randomized, dose-finding trial. British Journal of Dermatology 2005;152(6):1219-27. [CENTRAL: CN-00522446] - PubMed
Gottlieb 2006a {published data only}
-
- Gottlieb A, Zhang Y. A phase II trial of a new anti-inflammatory combination drug, CRx-140, in subjects with severe psoriasis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB8. [CENTRAL: CN-00602228]
Gottlieb 2006b {published data only}
-
- Gottlieb A, Cather J, Hamilton T, Sherman M. Preliminary clinical safety and efficacy results from an open-label phase 2 study of STA-5326, an oral IL-12/IL-23 inhibitor, in patients with moderate to severe chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB10. [CENTRAL: CN-00602166]
Gottlieb 2010 {published data only}
-
- Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373(9664):633-40. [CENTRAL: CN-00686924] - PubMed
Gottlieb 2016 {published data only}
-
- Gottlieb A, Gerdes S, Lacour J, Korman N, Papp K, Dutronic Y, et al. Efficacy of ixekizumab in moderate-to-severe psoriasis patients who have or have not received prior biologic therapies: an integrated analysis of 3 phase 3 studies. Journal of Investigative Dermatology 2016;136(9 Suppl 2):S169. [CENTRAL: CN-01747590]
Gottlieb 2017a {published data only}
-
- Gottlieb A, Sullivan J, Kubanov A, You R, Regnault P, Frueh J. Secukinumab shows high and sustained efficacy in patients with moderate-to-severe palmoplantar psoriasis: 2.5-year results from the GESTURE study. British Journal of Dermatology 2017;177(5):e261. [CENTRAL: CN-01452515] - PubMed
Gottlieb 2017b {published data only}
-
- Gottlieb A, Sullivan J, Van Doorn M, Kubanov A, You R, Parneix A, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. Journal of the American Academy of Dermatology 2017;76(1):70-80. [CENTRAL: CN-01368596] - PubMed
Gottlieb 2017c {published data only}
-
- Gottlieb AB, Merola JF, Chen R, Levi E, Duffin KC. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and Body Surface Area (PGA x BSA) composite tool: an analysis of apremilast phase 3 ESTEEM data. Journal of the American Academy of Dermatology 2017;77(6):1178-80. [CENTRAL: CN-01443069] - PubMed
Gottlieb 2017d {published data only}
-
- Gottlieb AB, Lacour JP, Korman N, Wilhelm S, Dutronc Y, Schacht A, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two Phase III randomized studies. Journal of the European Academy of Dermatology and Venereology 2017;31(4):679-85. [CENTRAL: CN-01244380] - PMC - PubMed
Gottlieb 2018a {published data only}
-
- Gottlieb AB, Gordon K, Hsu S, Elewski B, Eichenfield LF, Kircik L, et al. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. Journal of the European Academy of Dermatology and Venereology 2018;32(8):1305-13. [CENTRAL: CN-01572384] - PubMed
Gottlieb 2018b {published data only}
-
- Gottlieb AB, Blauvelt A, Thaçi D, Leonardi C, Poulin Y, Peterson L, et al. Durable reduction in absolute PASI with certolizumab pegol in patients with chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):24. [CENTRAL: CN-01620157]
Goupille 1995 {published data only}
-
- Goupille P, Valat JP. Is methotrexate really effective in patients with psoriatic arthritis? Journal of Rheumatology 1995;22(12):2369-70. [PMID: ] - PubMed
Goupille 2018 {published data only}
-
- Goupille P, Roussou E, Burmester G, Mease PJ, Gottlieb AB, Garces S, et al. Safety of ixekizumab in patients with psoriatic arthritis: results from a pooled analysis of three clinical trials. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1039-40. [DOI: 10.1136/annrheumdis-2018-eular.2132] - DOI
Griffiths 1998 {published data only}
-
- Griffiths CM, Boffa M, Wishart J, Adam C, Inglesias L, Van de Kerkhof P, et al. A double-blind, randomised trial to compare the effects of oral liarozole with acitretin in the treatment of chronic plaque psoriasis. British Journal of Dermatology 1998;139(Suppl 51):19. [CENTRAL: CN-00415772]
Griffiths 2002a {published data only}
-
- Griffiths CE, Humbert P, Koo J, Ortonne JP, Christophers E. Relationship between clinical response and quality of life in psoriasis patients treated with alefacept. Journal of the European Academy of Dermatology and Venereology 2002;16(Suppl S1):292. [CENTRAL: CN-00478562]
Griffiths 2002b {published data only}
-
- Griffiths CE, Ortonne JP, Christophers E. Effect of alefacept based on patients' response to prior therapy for psoriasis. British Journal of Dermatology 2002;147(Suppl 62):45. [CENTRAL: CN-00406976]
Griffiths 2005 {published data only}
-
- Griffiths CE. A higher treatment standard for patients with moderate to severe psoriasis. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):7. [CENTRAL: CN-00602410]
Griffiths 2010 {published data only}
-
- Griffiths CE, Menter A, Strober BE, Yeilding N. Ustekinumab treatment in patients with moderate to severe psoriasis who are nonresponders to etanercept: results from a phase III clinical trial. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB137. [CENTRAL: CN-00843739] [DOI: 10.1016/j.jaad.2009.11.527] - DOI
Griffiths 2016 {published data only}
-
- Griffiths CE, Warren R, Ilo D, Kerr L, Kent T, Mallbris L. Efficacy and safety of ixekizumab in patients with psoriasis who failed initial etanercept treatment: a subanalysis from UNCOVER 2, a randomized, double-blind, multicentre, phase III clinical trial. British Journal of Dermatology 2016;175(Suppl S1):68-9. [CENTRAL: CN-01303311] [DOI: 10.1111/bjd.14524] - DOI
Griffiths 2017 {published data only}
-
- Griffiths CE, Vender R, Sofen H, Kircik L, Tan H, Rottinghaus ST, et al. Effect of tofacitinib withdrawal and re-treatment on patient-reported outcomes: results from a phase 3 study in patients with moderate to severe chronic plaque psoriasis. Journal of the European Academy of Dermatology & Venereology 2017;31(2):323-32. [CENTRAL: CN-01332752] [DOI: 10.1111/jdv.13808] - DOI - PMC - PubMed
Griffiths 2018a {published data only}
-
- Griffiths CE, Papp KA, Kimball AB, Randazzo B, Song M, Li S, et al. Long-term efficacy of guselkumab for the treatment of moderate-to-severe psoriasis: results from the phase 3 VOYAGE 1 trial through two years. Journal of Drugs in Dermatology 2018;17(8):826-32. [CENTRAL: CN-01655581] - PubMed
Griffiths 2018b {published data only}
-
- Griffith C, Radtke MA, Youn SW, Bissonnette R, Song M, Wasfi Y, et al. Clinical response after guselkumab treatment among adalimumab PASI 90 non-responders: results from the Voyage 1 and 2 trials. Acta Dermato-Venereologica 2018;98(Suppl 219):20. [CENTRAL: CN-01620165]
Griffiths 2018c {published data only}
-
- Griffiths C, Blauvelt A, Reich K, Leonardi C, Mehta N, Tsai T, et al. Secukinumab's long-term safety remains favorable up to 5 years of treatment. Acta Dermato-Venereologica 2018;98(Suppl 219):46. [CENTRAL: CN-01920781]
Grim 2000 {published data only}
-
- Grim J, Chladek J, Martinkova J, Simkova M, Vaneckova J, Koudelkova V. Pharmacokinetics (PK) and pharmacodynamics (PD) of low dose methotrexate (LDMTX) in the treatment of psoriasis. British Journal of Clinical Pharmacology 2000;50(4):390-1. [EMBASE: 2000362454]
Grossman 1994 {published data only}
-
- Grossman RM, Thivolet J, Claudy A, Souteyrand P, Guilhou JJ, Thomas P, et al. A novel therapeutic approach to psoriasis with combination calcipotriol ointment and very low-dose cyclosporine: results of a multicenter placebo-controlled study. Journal of the American Academy of Dermatology 1994;31(1):68-74. [CENTRAL: CN-00102638] - PubMed
Guenther 2020 {published data only}
-
- Guenther L, Potts Bleakman A, Weisman J, Poulin Y, Spelman L, Burge R, et al. Ixekizumab results in persistent clinical improvement in moderate-to-severe genital psoriasis during a 52 week randomized, placebo-controlled, phase 3 clinical trial. Acta Dermato-Venereologica 2020;100(1):adv00006. - PMC - PubMed
Gulliver 1996 {published data only}
-
- Gulliver WP, Murphy GF, Hannaford VA, Primmett DR. Increased bioavailability and improved efficacy, in severe psoriasis, of a new microemulsion formulation of cyclosporin. British Journal of Dermatology 1996;135(s48):35-9. [EMBASE: 1996272533] - PubMed
Gupta 2005 {published data only}
-
- Gupta SK, Dogra A, Kaur G. Comparative efficacy of methotrexate and hydroxyurea in treatment of psoriasis. Journal of Pakistan Association of Dermatologists 2005;15(3):247-51. [CENTRAL: 2005580293]
Gupta 2007 {published data only}
-
- Gupta R, Gupta S. Methotrexate-betamethasone weekly oral pulse in psoriasis. Journal of Dermatological Treatment 2007;18(5):291-4. [CENTRAL: CN-00619338] - PubMed
Gupta 2008 {published data only}
-
- Gupta AK, Langley RG, Lynde C, Barber K, Gulliver W, Lauzon G, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. Journal of Cutaneous Medicine and Surgery 2008;12(6):268-75. [CENTRAL: CN-00683900] - PubMed
Han 2013 {published data only}
-
- Han C, Kavanaugh A, Genovese MC, Hsu B, Deodhar AA, Hsia EC. Sustained improvement in health-related quality of life, work productivity, employability, and reduced healthcare resource utilization of patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis treated with golimumab: 5-year results from 3 phase III studies. Arthritis and Rheumatism 2013;65(S10):S137. [CENTRAL: CN-01062782]
Hashizume 2007 {published data only}
-
- Hashizume H, Ito T, Yagi H, Takigawa M, Kageyama H, Furukawa F, et al. Efficacy and safety of preprandial versus postprandial administration of low-dose cyclosporin microemulsion (Neoral) in patients with psoriasis vulgaris. Journal of Dermatology 2007;34(7):430-4. [CENTRAL: CN-00610280] - PubMed
Hawkes 2018 {published data only}
-
- Hawkes JE, Lebwohl M, Elewski B, Kircik L, Reich K, Muscianisi E, et al. Secukinumab for the treatment of scalp, nail, and palmoplantar psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S28.
Heule 1988 {published data only}
-
- Heule F, Meinardi MM, Van Joost T, Bos JD. Low-dose cyclosporine effective in severe psoriasis: a double-blind study. Transplantation Proceedings 1988;20(3 Suppl 4):32-41. [CENTRAL: CN-00054273] - PubMed
Ho 2010 {published data only}
-
- Ho SG, Yeung CK, Chan HH. Methotrexate versus traditional Chinese medicine in psoriasis: a randomized, placebo-controlled trial to determine efficacy, safety and quality of life. Clinical and Experimental Dermatology 2010;35(7):717-22. [CENTRAL: CN-00761451] - PubMed
Holzer 2020 {published data only}
-
- Holzer G, Hoke M, Sabeti-Sandor S, Perkmann T, Rauscher A, Strassegger B, et al. Disparate effects of adalimumab and fumaric acid esters on cardiovascular risk factors in psoriasis patients: results from a prospective, randomized, observer-blinded head-to-head trial. Journal of the European Academy of Dermatology and Venereology 2020;35(2):441-9. - PubMed
Hsu 2018 {published data only}
-
- Hsu S, Green L, Keegan BR, Kircik L, Rastogi S, Pillai R, et al. Efficacy of brodalumab in ustekinumab-naive and-experienced patients with moderate-to-severe plaque psoriasis. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S27.
Hunter 1972 {published data only}
-
- Hunter GA, Simmons IJ, Thomas BM. A clinical trial of hydroxyurea for psoriasis. Australasian Journal of Dermatology 1972;13(3):93-9. [CENTRAL: CN-00008809] - PubMed
Iest 1989 {published data only}
-
- Iest J, Boer J. Combined treatment of psoriasis with acitretin and UVB phototherapy compared with acitretin alone and UVB alone. British Journal of Dermatology 1989;120(5):665-70. [CENTRAL: CN-00568569] - PubMed
Imafuku 2017 {published data only}
-
- Imafuku S, Torisu-Itakura H, Nishikawa A, Zhao F, Cameron GS, Japanese Uncover-Study Group. Efficacy and safety of ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis: subgroup analysis of a placebo-controlled, phase 3 study (UNCOVER-1). Journal of Dermatology 2017;44(11):1285-90. [CENTRAL: CN-01615794] - PMC - PubMed
ISRCTN18043449 {published data only}
-
- ISRCTN18043449. A study of pre-clinical joint disease in psoriasis and the imaging response to ustekinumab. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN18043449 (first received 19 September 2012).
Iversen 2018 {published data only}
-
- Iversen L, Eidsmo L, Austad J, De Rie M, Osmancevic A, Skov L, et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification - rationale and design of the randomized, multicenter STEPIn study. Journal of the European Academy of Dermatology and Venereology 2018;32(11):1930-9. [CENTRAL: CN-01630270] - PubMed
Jackson 2018 {published data only}
-
- Jackson JM, Alikhan A, Lebwohl M, Stein Gold L, Levi E, Bagel J. Improvement in scalp and nails with apremilast in patients with moderate plaque psoriasis naive to systemic and biologic therapy: 52-week results of the UNVEIL study. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S20-1.
Jacobe 2008 {published data only}
-
- Jacobe H, Winterfield L, Kim F, Huet-Adams B, Cayce R. The role of narrowband UV-B plus alefacept combination therapy in the treatment of psoriasis. Archives of Dermatology 2008;144(8):1067-8; author reply 1068-9. [CENTRAL: CN-00650560] [PMID: ] - PubMed
JapicCTI‐194706 2019 {published data only}
-
- JPRN-JapicCTI-194706. A multicenter, randomized, open-label study to evaluate the safe and effective use of the prefilled safety syringe or the auto-injector for the subcutaneous self-injection of bimekizumab solution by subjects with moderate to severe chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-194706 (first received 11 April 2019).
Jin 2017 {published data only}
-
- Huang YW, Tsai TF. Efficacy of tofacitinib in patients with moderate-to-severe psoriasis who had inadequate responses to prior biologics. Dermatologica Sinica 2019;37(4):205-8.
-
- Jin T, Sun Z, Chen X, Wang Y, Li R, Ji S, et al. Serum human beta-defensin-2 is a possible biomarker for monitoring response to JAK inhibitor in psoriasis patients. Dermatology 2017;233(2-3):164-9. [CENTRAL: CN-01622175] - PubMed
Joergensen 2022 {published data only}
-
- Joergensen K K, Syversen S W, Goll G L, Bjrlykke K H, Sandanger O, Sexton J, Brun M K, Mrk C, Kvien T K, Gehin J E, Olsen I C, Bolstad N, Haavardsholm E A, Jahnsen J. Proactive therapeutic drug monitoring is superior to standard treatment during maintenance therapy with infliximab; results from a 52-week multicentre randomised trial of 450 patients. Gastroenterology 2022;162(7 Suppl):S-44.
JPRN‐jRCT2071210135 {published data only}
-
- JPRN-jRCT2071210135. Bioequivalence of DMB-3115, a usutekinumab biosimilar, and usutekinumab. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-jRCT2071210135 (first received 22 March 2022).
JPRN‐jRCTs041180012 2018 {published data only}
-
- JPRN-jRCTs041180012. Comparison of phototherapy alone or together with apremilast in psoriasis vulgaris patients. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs041180012 (first received 9 November 2018).
Kaur 2018 {published data only}
-
- Kaur S, Shafiq N, Dogra S, Mittal B, Bahl A, Narang T, et al. A placebo-controlled, randomized trial to assess, using pet scan, systemic and vascular inflammation, and response to therapy, in patients with psoriasis. Journal of the Dermatology Nurses' Association (Conference: 24th World Congress of Dermatology, Italy) 2020;12(2):No-pagination.
-
- Kaur S, Shafiq N, Dogra S, Mittal BR, Attri SV, Bahl A, et al. 18F-fluorodeoxyglucose positron emission tomography-based evaluation of systemic and vascular inflammation and assessment of the effect of systemic treatment on inflammation in patients with moderate-to-severe psoriasis: a randomized placebo-controlled pilot study. Indian Journal of Dermatology, Venereology and Leprology 2018;84(6):660-6. [CENTRAL: CN-01670374] - PubMed
Kavanaugh 2009 {published data only}
-
- Kavanaugh A. The efficacy of ustekinumab on the articular and dermatologic manifestations of psoriatic arthritis. Current Rheumatology Reports 2009;11(4):233-4. [CENTRAL: CN-00958869] - PubMed
Kemeny 2019 {published data only}
-
- Kemeny L, Berggren L, Dossenbach M, Dutronc Y, Paul C. Efficacy and safety of ixekizumab in patients with plaque psoriasis across different degrees of disease severity: results from UNCOVER-2 and UNCOVER-3. Journal of Dermatological Treatment 2019;30(1):19-26. [CENTRAL: CN-01913619] - PubMed
Kimball 2008 {published data only}
-
- Kimball AB, Gordon KB, Langley RG, Menter A, Chartash EK, Valdes J, et al. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Archives of Dermatology 2008;144(2):200-7. [CENTRAL: CN-00630180] - PubMed
Kimball 2011 {published data only}
-
- Kimball AB, Gordon KB, Langley RG, Menter A, Perdok RJ, Valdes J. Efficacy and safety of ABT-874, a monoclonal anti-interleukin 12/23 antibody, for the treatment of chronic plaque psoriasis: 36-week observation/retreatment and 60-week open-label extension phases of a randomized phase II trial. Journal of the American Academy of Dermatology 2011;64(2):263-74. [CENTRAL: CN-00770794] - PubMed
Kimball 2018 {published data only}
-
- Kimball AB, Luger T, Gottlieb A, Puig L, Kaufmann R, Burge R, et al. Long-term impact of ixekizumab on psoriasis itch severity: results from a phase III clinical trial and long-term extension. Acta Dermato-Venereologica 2018;98(1):98-102. [CENTRAL: CN-01446779] - PubMed
Kohm 2022 {published data only}
-
- Kohm M, Rossmanith T, Foldenauer AC, Kellner H, Kiltz U, Rech J, et al. Efficacy of UST in active PSA is independent from concomitant MTX use, even in patients with more severe skin psoriasis: subgroup analysis from a randomized placebo-controlled investigator initiated clinical trial. Annals of the Rheumatic Diseases 2022;81(Suppl 1):850-1.
Koo 1998 {published data only}
-
- Koo J, OLP302 Study Group. A randomized, double-blind study comparing the efficacy, safety and optimal dose of two formulations of cyclosporin, Neoral and Sandimmun, in patients with severe psoriasis. British Journal of Dermatology 1998;139(1):88-95. [CENTRAL: CN-00155452] - PubMed
Kopp 2015 {published data only}
-
- Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 2015;521(7551):222-6. [CENTRAL: CN-01074739] - PubMed
Korotaeva 2021 {published data only}
-
- Korotaeva TV, Mazurov VI, Lila AM, Gaydukova IZ, Bakulev AL, Samtsov AV, et al. Efficacy of netakimab in key psoriatic arthritis domains: 54-week results from the phase III BCD-085-8/patera study. Nauchno-Prakticheskaya Revmatologiya 2021;59(1):47-55.
Kragballe 1989 {published data only}
-
- Kragballe K, Jansén CT, Geiger JM, Bjerke JR, Falk ES, Gip L, et al. A double-blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicentre study. Acta Dermato-Venereologica 1989;69(1):35-40. [CENTRAL: CN-00057941] - PubMed
Krishnan 2005 {published data only}
-
- Krishnan KR, Cella D, Woolley M, Lalla D, Zitnik R, Brajac D. Etanercept improves symptoms of depression and fatigue in patients with psoriasis. In: 158th Annual Meeting of the American Psychiatric Association; 2005 May 21-26; Atlanta, GA. Vol. 158. 2005:NR293. [CENTRAL: CN-00595903]
Krishnan 2018 {published data only}
-
- Krishnan E, Zhang N, Wang H. Injection site reactions and injection site pain for the adalimumab biosimilar ABP 501: results from two double-blind randomized controlled studies. United European Gastroenterology Journal 2018;6(8 Suppl):A451. [CENTRAL: CN-01787637]
Kristensen 2017 {published data only}
-
- Kristensen LE, Merola JF, Dutz J, Adams DH, Kerr L, Rich P. Ixekizumab improves nail and skin lesions in patients with active psoriatic arthritis and prior TNF inadequate response. Annals of the Rheumatic Diseases 2017;76(Suppl 2):937. [CENTRAL: CN-01467783]
Krueger 1980 {published data only}
-
- Krueger GG, Shelby NJ, Hansen CD, Taylor MB. Comparison of labelling indices of skin involved and uninvolved with psoriasis: placebo and oral retinoid RO 10-9359 vs. time. Clinical Research 1980;28(1):21A. [CENTRAL: CN-00192437]
Krueger 2002a {published data only}
-
- Feldman SR, Menter A, Koo JY. Improved health-related quality of life following a randomized controlled trial of alefacept treatment in patients with chronic plaque psoriasis. British Journal of Dermatology 2004;150(2):317-26. [CENTRAL: CN-00471158] [PMID: ] - PubMed
-
- Krueger GG, Papp KA, Stough DB, Loven KH, Gulliver WP, Ellis CN, et al. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. Journal of the American Academy of Dermatology 2002;47(6):821-33. [CENTRAL: CN-00411712] [PMID: ] - PubMed
-
- Krueger GG. Clinical response to alefacept: results of a phase 3 study of intravenous administration of alefacept in patients with chronic plague psoriasis. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 2):17-24. [CENTRAL: CN-00456895] [PMID: ] - PubMed
-
- Menter A, Cather JC, Baker D, Farber HF, Lebwohl M, Darif M. The efficacy of multiple courses of alefacept in patients with moderate to severe chronic plaque psoriasis. Journal of the American Academy of Dermatology 2006;54(1):61-3. [CENTRAL: CN-00622887] [EMBASE: 2005584777] - PubMed
Krueger 2002b {published data only}
-
- Krueger G, Vaishnaw A, Rizova E. Pharmacodynamic effects of IM or IV alefacept: selective reductions in memory- effector (CD45RO+) cells are related to clinical improvement in psoriasis. Journal of Investigative Dermatology 2002;118(6):1098. [CENTRAL: CN-00795004]
Krueger 2003 {published data only}
-
- Krueger GG, Gordon KB, Van de Kerkhof P, Sterry W. Repeated courses of IM alefacept in psoriasis: rationale and design of an international study that mimics the clinical practice setting. Journal of Investigative Dermatology 2003;121(2):57. [CENTRAL: CN-00550788]
Krueger 2012 {published data only}
Krueger 2015 {published data only}
-
- Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. Journal of Allergy and Clinical Immunology 2015;136(1):116-24.e7. [CENTRAL: CN-01110170] [PMID: ] - PubMed
Krueger 2016a {published data only}
-
- Krueger J, Clark JD, Suarez-Farinas M, Fuentes-Duculan J, Cueto I, Wang CQ, et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. Journal of Allergy and Clinical Immunology 2016;137(4):1079-90. [CENTRAL: CN-01153710] [PMID: ] - PubMed
Krueger 2016b {published data only}
-
- Krueger JG, Wharton K, Schlitt T, Torene R, Jiang X, Wang CQ, et al. Secukinumab, a new anti-IL17A biologic therapy, induces rapid and durable clinical, histological, and molecular resolution of psoriasis plaques over 1 year of administration. Experimental Dermatology 2016;25(Suppl 4):26.
Krueger 2022 {published data only}
-
- Krueger J, Langley R, Nigen S. 35098 Secukinumab versus guselkumab in the treatment of ustekinumab-resistant psoriatic plaques: 16-week randomized, open-label, multicenter ARROW study. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB108.
Krupashankar 2014 {published data only}
-
- Dogra S, Krupashankar DS, Budamakuntla L, Srinivas CR, Khopkar U, Gupta S, et al. Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: a double-blind, randomized-withdrawal, placebo-controlled study. Journal of the American Academy of Dermatology 2015;73(2):331-3.e1. [CENTRAL: CN-01108850] [PMID: ] - PubMed
-
- Krupashankar DS, Dogra S, Kura M, Saraswat A, Budamakuntla L, Sumathy TK, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. Journal of the American Academy of Dermatology 2014;71(3):484-92. [CENTRAL: CN-01002561] [PMID: ] - PubMed
Kuijpers 1998 {published data only}
-
- Kuijpers AL, Van Pelt JP, Bergers M, Boegheim PJ, Den Bakker JE, Siegenthaler G, et al. The effects of oral liarozole on epidermal proliferation and differentiation in severe plaque psoriasis are comparable with those of acitretin. British Journal of Dermatology 1998;139(3):380-9. [CENTRAL: CN-00159672] - PubMed
Lajevardi 2015 {published data only}
-
- Lajevardi V, Hallaji Z, Daklan S, Abedini R, Goodarzi A, Abdolreza M. The efficacy of methotrexate plus pioglitazone vs. methotrexate alone in the management of patients with plaque-type psoriasis: a single-blinded randomized controlled trial. International Journal of Dermatology 2015;54(1):95-101. [CENTRAL: CN-01052011] - PubMed
Lambert 2018 {published data only}
-
- Lambert J, Ghislain P-D, Merola J, Potts-Bleakman A, Brnabic AJ, Burge R, et al. Clinical signs of epithelial surface disruption impact pain and sexual health in patients with moderate-to-severe genital psoriasis. British Journal of Dermatology 2018;179(Suppl 1):36. [CENTRAL: CN-01620064]
Langewouters 2005 {published data only}
-
- Langewouters AM, Bovenschen HJ, De Jong EM, Van Erp PJM, Van de Kerkhof PC. The effect of topical corticosteroids in combination with alefacept on circulating T-cell subsets in psoriasis. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):240. [CENTRAL: CN-00602421] - PubMed
Langley 2006 {published data only}
-
- Langley R, Leondardi C, Okun M. Long-term safety and efficacy of adalimumab in psoriasis. In: 4th European Association of Dermatology and Venereology (EADV) Spring Symposium Saariselka, Lapland, Finland. February 9-12th, 2006. Vol. Suppl. 2006:P-021. [CENTRAL: CN-00602234]
Langley 2010 {published data only}
-
- Langley RG, Papp K, Bissonnette R, Toth D, Matheson R, Hultquist M, et al. Safety profile of intravenous and subcutaneous siplizumab, an anti-CD2 monoclonal antibody, for the treatment of plaque psoriasis: results of two randomized, double-blind, placebo-controlled studies. International Journal of Dermatology 2010;49(7):818-28. [CENTRAL: CN-00761682] - PubMed
Langley 2016 {published data only}
-
- Langley R, Feldman S, Paul C, Gordon K, Strand V, Toth D, et al. Treatment with ixekizumab over 60 weeks provides sustained improvements in healthrelated quality of life: results from UNCOVER-1, a randomized phase 3 trial. Journal of Investigative Dermatology 2016;136(9 Suppl 2):S169. [CENTRAL: CN-01383388]
Langley 2018 {published data only}
-
- Langley RG, Tsai TF, Flavin S, Song M, Randazzo B, Wasfi Y, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. British Journal of Dermatology 2018;178(1):114-23. [CENTRAL: CN-01421735] [DOI: 10.1111/bjd.15750] - DOI - PubMed
Langner 2004 {published data only}
-
- Langner A, Roszkiewicz J, Baran E, Placek W. Results of a phase II study of a novel oral fumarate, BG-12, in the treatment of severe psoriasis. Journal of the European Academy of Dermatology and Venereology 2004;18(6):798. [CENTRAL: CN-00550917]
Lauharanta 1989 {published data only}
-
- Lauharanta J, Geiger JM. A double-blind comparison of acitretin and etretinate in combination with bath PUVA in the treatment of extensive psoriasis. British Journal of Dermatology 1989;121(1):107-12. [CENTRAL: CN-00061540] - PubMed
Lawrence 1983 {published data only}
-
- Lawrence CM, Marks J, Shuster S. Addition of retinoids to PUVA for psoriasis. Lancet 1983;1(8326 Pt 1):706. [CENTRAL: CN-00030597] - PubMed
Leavell 1970 {published data only}
-
- Leavell UW, Yarbro JW. Hydroxyurea. A new treatment for psoriasis. Archives of Dermatology 1970;102(2):144-50. [CENTRAL: CN-00004668] - PubMed
Lebwohl 2003 {published data only}
-
- Finlay AY, Salek MS, Haney J, Alefacept Clinical Study Group. Intramuscular alefacept improves health-related quality of life in patients with chronic plaque psoriasis. Dermatology (Basel, Switzerland) 2003;206(4):307-15. [CENTRAL: CN-00437818] [PMID: ] - PubMed
-
- Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Archives of Dermatology 2003;139(6):719-27. [CENTRAL: CN-00438439] [PMID: ] - PubMed
-
- Ortonne JP, Lebwohl M, Griffiths CE. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. European Journal of Dermatology 2003;13(2):117-23. [CENTRAL: CN-00436640] [PMID: ] - PubMed
-
- Ortonne JP. Clinical response to alefacept: results of a phase 3 study of intramuscular administration of alefacept in patient with chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 2):12-6. [CENTRAL: CN-00456894] [PMID: ] - PubMed
Lebwohl 2003a {published data only}
-
- Lebwohl M. The effect of psoriasis and its treatments on circulating T-cell subsets: results of alefacept studies. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):377. [CENTRAL: CN-00478640]
Lebwohl 2009 {published data only}
-
- Lebwohl M, Kimball A, Gordon K, Szapary P. Comparable efficacy and safety of ustekinumab in moderate to severe psoriasis patients previously treated with systemic therapies and treatment-naive patients. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB167.
Lebwohl 2012 {published data only}
-
- Lebwohl MG, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, et al. Safety and efficacy of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. Dermatology and Therapy 2012;2:S39. [CENTRAL: CN-01027857] - PubMed
Lebwohl 2013 {published data only}
-
- Lebwohl MG, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2013;69(3):385-92. [CENTRAL: CN-00964028] - PubMed
Ledo 1988 {published data only}
-
- Ledo A, Martin M, Geiger JM, Marron JM. Acitretin (Ro 10-1670) in the treatment of severe psoriasis. A randomized double-blind parallel study comparing acitretin and etretinate. International Journal of Dermatology 1988;27(9):656-60. [CENTRAL: CN-00058382] - PubMed
Legat 2005 {published data only}
-
- Legat LJ, Hofer A, Wackernagel A, Salmhofer W, Kerl H, Wolf P. Alefacept plus 311 nm narrowband ultraviolet B (NB-UVB) phototherapy in the treatment of psoriasis. Journal of Investigative Dermatology 2005;125(1):A4. [CENTRAL: CN-00550842]
Leonardi 2010a {published data only}
-
- Leonardi C, Guenther L, Wasel N, Yeilding N, Szapary PO, Hsu MC, et al. Characterization of infections associated with ustekinumab in moderate to severe psoriasis patients. Journal of the European Academy of Dermatology and Venereology 2010;24(Suppl 4):22. [EMBASE: 70238720]
Leonardi 2010b {published data only}
-
- Leonardi C, Menter A, Gu Y, Okun M. Efficacy and safety of weekly adalimumab in psoriasis patients with a less than PASI 50 response to 40 mg every other week: results from an open-label extension study. Journal of the European Academy of Dermatology and Venereology 2010;24(Suppl 4):9-10. [EMBASE: 70238693]
Leonardi 2010c {published data only}
-
- Leonardi C, Papp K, Asahina A, Gu Y, Rozzo S. Long-term safety of adalimumab for psoriasis: an analysis of all adalimumab exposure in all global clinical trials. Journal of the European Academy of Dermatology and Venereology 2010;24:26. [CENTRAL: 70238729]
Leonardi 2011a {published data only}
-
- Leonardi C, Kimball A, Schenkel B, Papp K. Sustained improvement in skin disease specific quality of life in patients with moderate to severe psoriasis receiving ustekinumab maintenance therapy: long-term results from PHOENIX 1. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB149. [CENTRAL: CN-00843843] [DOI: 10.1016/j.jaad.2010.09.608] - DOI
Leonardi 2011b {published data only}
-
- Leonardi C, Langley RG, Papp K, Tyring SK, Wasel N, Vender R, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Archives of Dermatology 2011;147(4):429-36. [CENTRAL: CN-00785710] - PubMed
Levell 1995 {published data only}
-
- Levell NJ, Shuster S, Munro CS, Friedmann PS. Remission of ordinary psoriasis following a short clearance course of cyclosporin. Acta Dermato-Venereologica 1995;75(1):65-9. [CENTRAL: CN-00113972] - PubMed
Li 2018 {published data only}
-
- Li N, Teeple A, Muser E, McElligott S, You Y, Song M, et al. Work/study productivity gain and indirect cost savings with guselkumab compared with adalimumab in moderate to severe psoriasis: results from the VOYAGE 1 study. Journal of Managed Care and Specialty Pharmacy 2018;24(10 A):S81. [CENTRAL: CN-01670028] - PubMed
Li 2022 {published data only}
Liang 1995 {published data only}
-
- Liang GS, Kerdel FA. Combination therapy and the use of an initial dose of intramuscular methotrexate in patients hospitalized for psoriasis. Journal of Dermatological Treatment 1995;6(2):73-6. [CENTRAL: CN-00171665]
Louw 2017 {published data only}
-
- Louw I, Kivitz AJ, Takeuchi T, Tanaka Y, Nakashima S, Hodge J, et al. The long-term safety and durability of response of CHS-0214, a proposed biosimilar to etanercept: an open-label safety extension study. Arthritis and Rheumatology 2017;69(Suppl 10):2492. [CENTRAL: CN-01423778]
Lui 2011 {published data only}
-
- Lui H, Tan J, Shear N, Bissonnette R, Gulliver W. Efficacy and safety of alefacept in combination with narrowband UVB compared to alefacept alone in subjects with moderate to severe psoriasis: results of the Canadian alefacept phototherapy psoriasis study. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN-00843846]
Lui 2012 {published data only}
-
- Lui H, Gulliver W, Tan J, Hong CH, Hull P, Shear NH, et al. A randomized controlled study of combination therapy with alefacept and narrow band UVB phototherapy (UVB) for moderate to severe psoriasis: efficacy, onset, and duration of response. Journal of Drugs in Dermatology 2012;11(8):929-37. [CENTRAL: CN-01164684] - PubMed
Lynde 2012 {published data only}
-
- Lynde CW, Gupta AK, Guenther L, Poulin Y, Levesque A, Bissonnette R. A randomized study comparing the combination of nbUVB and etanercept to etanercept monotherapy in patients with psoriasis who do not exhibit an excellent response after 12 weeks of etanercept. Journal of Dermatological Treatment 2012;23(4):261-7. [CENTRAL: CN-00972294] [PMID: ] - PubMed
Macdonald 1972 {published data only}
-
- Macdonald A, Fry L. Retinoic acid in the treatment of psoriasis. British Journal of Dermatology 1972;86(5):524-7. [CENTRAL: CN-00007340] - PubMed
Mahrle 1995 {published data only}
-
- Mahrle G, Schulze HJ, Farber L, Weidinger G, Steigleder GK. Low-dose short-term cyclosporine versus etretinate in psoriasis: improvement of skin, nail, and joint involvement. Journal of the American Academy of Dermatology 1995;32(1):78-88. [CENTRAL: CN-00109143] - PubMed
Malik 2010 {published data only}
-
- Malik T, Ejaz A. Comparison of methotrexate and azathioprine in the treatment of psoriasis: a randomized controlled trial. Journal of Pakistan Association of Dermatologists 2010;20(3):152-7. [CENTRAL: CN-00789615]
Marecki 2004 {published data only}
-
- Marecki S, Kirkpatrick P. Efalizumab. Nature Reviews. Drug Discovery 2004;3(6):473-4. [PMID: ] - PubMed
Marks 1986 {published data only}
-
- Marks JM. Cyclosporin A treatment of severe psoriasis. British Journal of Dermatology 1986;115(6):745-6. [PMID: ] - PubMed
Mate 2017 {published data only}
-
- Mate E, Bagel J, Callis-Duffin K, Moore A, Ferris L, Siu K, et al. Secukinumab is efficacious in clearing moderate-tosevere scalp psoriasis: 12 week results of a randomized phase IIIb study. Australasian Journal of Dermatology 2017;58(Suppl 1):71. [CENTRAL: CN-01378822]
Mate 2018 {published data only}
-
- Mate E, Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile through 5 years of treatment in moderate to severe psoriasis. Australasian Journal of Dermatology 2018;59(Suppl 1):86. [CENTRAL: CN-01919964] - PMC - PubMed
McInnes 2013 {published data only}
-
- McInnes IB, Papp K, Puig L, Reich K, Ritchlin CT, Strober B, et al. Safety of ustekinumab from the placebo-controlled periods of psoriatic arthritis and psoriasis clinical developmental programs. Annals of the rheumatic diseases 2013;72(Suppl 3):No pagination. [DOI: 10.1136/annrheumdis-2013-eular.1992] - DOI
McInnes 2017 {published data only}
Mease 2011 {published data only}
-
- Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis and Rheumatism 2011;63(4):939-48. [CENTRAL: CN-00779390] - PubMed
Mease 2016a {published data only}
-
- Mease PJ, Okada M, Kishimoto M, Shuler CL, Carlier H, Lin CY, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52 week results from a phase 3 study. Arthritis and Rheumatology 2016;68(Suppl 10):1270-1. [CENTRAL: CN-01296553] - PubMed
Mease 2016b {published data only}
-
- Mease P, Van Der Heijde D, Ritchlin C, Cuchacovich R, Shuler C, Lin CY, et al. A randomized, double-blind, active- and placebo-controlled phase 3 study of efficacy and safety of ixekizumab, adalimumab, and placebo therapy in patients naive to biologic disease modifying antirheumatic drugs with active psoriatic arthritis. Journal of Rheumatology 2016;43(6):1169. [CENTRAL: CN-01294588]
Mease 2017a {published data only}
-
- Mease PJ, Van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Annals of the Rheumatic Diseases 2017;76(1):79-87. [CENTRAL: CN-01374947] - PMC - PubMed
Mease 2017b {published data only}
-
- Mease P, Kishimoto M, Okada M, Lee C, Moriarty S, Mou J, et al. 52-week efficacy and safety results from SPIRIT-P1: a phase 3 study of ixekizumab in patients with active psoriatic arthritis. Journal of Rheumatology 2017;44(6):925. [CENTRAL: CN-01398036] - PubMed
Mease 2017c {published data only}
-
- Mease P, Okada M, Kishimoto M, Shuler C, Carlier H, Lin C, et al. Fifty-two-week efficacy and safety results from SPIRIT-P1: a phase 3 study of ixekizumab in patients with active psoriatic arthritis. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S260. [CENTRAL: CN-01416618]
Mease 2018 {published data only}
-
- Mease P, Van der Heijde D, Landewe R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Annals of the Rheumatic Diseases 2018;77(6):890-7. [CENTRAL: CN-01606299] - PMC - PubMed
Mease 2020 {published data only}
-
- Mease PJ, Chohan S, Garcia Fructuoso FJ, Gottlieb AB, Chou R, Mendelsohn AM, et al. 15964 Randomized, double-blind, placebo-controlled, multiple-dose, phase 2b study to demonstrate the safety and efficacy of tildrakizumab, a high-affinity anti-interleukin-23p19 monoclonal antibody, in patients with active psoriatic arthritis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB165.
Mease 2022 {published data only}
-
- Mease P J, Foley P, Reich K, Chakravarty SD, Shawi M, Yang YW, et al. Targeted safety analyses of guselkumab: long-term results from randomized clinical trials in patients with active psoriatic arthritis and moderate to severe psoriasis. Annals of the Rheumatic Diseases 2022;81(Suppl 1):1572-3.
Meffert 1989 {published data only}
-
- Meffert H, Sönnichsen N. Acitretin in the treatment of severe psoriasis: a randomized double-blind study comparing acitretin and etretinate. Acta Dermato-Venereologica 1989;146(Suppl):176-7. [CENTRAL: CN-00064910] - PubMed
Menon 2012 {published data only}
-
- Menon S, Boy MG, Wang C, Wilkinson BE, Zwillich SH, Chan G, et al. Single and multiple-dose pharmacokinetics of tofacitinib (CP-690,550) from a double-blind, placebo-controlled, dose-escalation study in medically stable subjects with psoriasis. Clinical Pharmacology and Therapeutics 2012;91:S33. [CENTRAL: CN-01034861]
Menter 2007 {published data only}
-
- Menter A, Guzzo C, Li S, Gottlieb AB. Efficacy of infliximab in patients with severe psoriasis: subgroup analysis from clinical trials. Journal of the American Academy of Dermatology 2007;56(2):AB174. [CENTRAL: CN-00615988]
Menter 2014 {published data only}
-
- Menter A, Papp KA, Tan H, Tyring S, Wolk R, Buonanno M. Efficacy of tofacitinib, an oral janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. Journal of Drugs in Dermatology 2014;13(3):252-6. [CENTRAL: CN-00985274] - PubMed
Menter 2022 {published data only}
-
- Menter A, McCabe D, Lang B. 33221 Further clinical outcomes in patients with moderate-to-severe chronic plaque psoriasis receiving adalimumab reference product continuously or switching between BI 695501 and adalimumab RP in the phase III, randomized, interchangeability VOLTAIRE-X t. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB61.
Merola 2017 {published data only}
-
- Merola JF, Elewski B, Tatulych S, Lan S, Tallman A, Kaur M. Efficacy of tofacitinib for the treatment of nail psoriasis: two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2017;77(1):79-87.e1. [CENTRAL: CN-01401051] - PubMed
Merola 2018 {published data only}
-
- Merola JF, Kishimoto M, Adams D, Park SY, Thaçi D. Ixekizumab improves nail and skin psoriasis through 52 weeks of treatment in patients with active psoriatic arthritis: results from two randomized, double-blind, phase 3, clinical trials (SPIRIT-P1 and SPIRIT-P2). Acta Dermato-Venereologica 2018;98(Suppl 219):16. [CENTRAL: CN-01620172]
Merola 2020 {published data only}
-
- Merola JF, Ghislain P-D, Dauendorffer JN, Potts Bleakman A, Brnabic AJM, Burge R, et al. Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis. Journal of the European Academy of Dermatology and Venereology 2020;34(6):1257-62. - PMC - PubMed
-
- Merola JF, Gottlieb AB, Mease P, Strand V, Kavanaugh A, Liu L, et al. 13350 Psoriasis outcomes in a randomized trial of etanercept and methotrexate as monotherapy or in combination in patients with psoriatic arthritis. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB118.
Meyer 2011 {published data only}
-
- Meyer MW, Zachariae C, Bendtzen K, Skov L. Immunogenicity of tumour necrosis factor inhibitors in patients with psoriasis receiving long-term treatment. British Journal of Dermatology 2011;165(6):e17. [EMBASE: 70610785]
Mittal 2009 {published data only}
-
- Mittal R, Malhotra S, Pandhi P, Kaur I, Dogra S. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Archives of Dermatology 2009;145(4):387-93. [CENTRAL: CN-00682023] - PubMed
Moller 2009 {published data only}
-
- Moller I. Efficacy of leflunomide in patients with psoriatic arthritis [Eficacia del tratamiento con leflunomida en pacientes con artritis psoriasica]. Seminarios de la Fundacion Espanola de Reumatologia 2009;10(2):48-52. [PMID: ] - PubMed
Monk 1986 {published data only}
-
- Monk BE. Cyclosporin A and psoriasis. British Journal of Dermatology 1986;115(2):249-50. [PMID: ] - PubMed
Montgomery 1993 {published data only}
-
- Montgomery JA, Snyder HW Jr, Walsh DA, Walsh GM. BCX-34. Purine nucleoside phosphorylase (PNP) inhibitor. Drugs of the Future 1993;18(10):887-90. [EMBASE: 1994025323]
Mrowietz 1991 {published data only}
-
- Mrowietz U, Christophers E. Low-dose ciclosporin A (sandimmun) in psoriasis: a multicenter dose-finding study. Zeitschrift fur Hautkrankheiten 1991;66(Suppl 1):25-9. [CENTRAL: CN-00182853]
Mrowietz 2012 {published data only}
-
- Mrowietz U, Reich K, Rozzo S, Gu Y. Achievement of European Consensus Programme treatment goals in three clinical trials of adalimumab in moderate-to-severe psoriasis. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB183. [EMBASE: 70704582]
Narang 2012 {published data only}
-
- Narang T, Dogra S, Handa S. Serendipity opens new avenues: a pilot study to evaluate the efficacy of saxagliptin in combination with cyclosporine and acitretin in diabetic psoriasis patients. Dermatology and Therapy 2012;2(Suppl 1):S36-7. [CENTRAL: CN-01027858]
Nash 2015 {published data only}
-
- Nash P, Gottlieb A, Mease P, McInnes I, Kirkham B, Kavanaugh A, et al. Secukinumab, a human anti-interleukin-17a monoclonal antibody, significantly reduces psoriasis burden in patients with psoriatic arthritis: results from a phase 3 randomized controlled trial. Internal Medicine Journal 2015;45(Suppl 2):42. [CENTRAL: CN-01361215]
NCT00106847 {published data only}
-
- NCT00106847. A study of the safety and effectiveness of infliximab in patients with plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT00106847 (first received 1 April 2005).
NCT00111111 {published data only}
-
- NCT00111111. An evaluation of etanercept in the treatment of subjects with psoriasis. clinicaltrials.gov/ct2/show/nct00111111 (first received 18 May 2005).
NCT00258713 {published data only}
-
- NCT00258713. A 36-week extension to protocol ISA04-03. clinicaltrials.gov/ct2/show/NCT00258713 (first received 28 November 2005).
NCT00358670 {published data only}
-
- NCT00358670. Long-term effects of infliximab in the treatment of moderate to severe psoriasis [extension of study P04271, NCT00251641] (P04563). clinicaltrials.gov/ct2/show/nct00358670 (first received 1 August 2006).
NCT00377325 {published data only}
-
- NCT00377325. The effectiveness of lower cyclosporine doses for psoriasis. clinicaltrials.gov/show/nct00377325 (first received 18 September 2006).
NCT00438360 {published data only}
-
- NCT00438360. Efficacy and safety of cyclosporine A microemulsion in maintenance patients with chronic plaque psoriasis. clinicaltrials.gov/show/nct00438360 (first received 22 February 2007).
NCT00585650 {published data only}
-
- NCT00585650. Study of tumor necrosis factor receptor fusion protein etanercept (enbrel) in psoriasis of the hands and/or feet. clinicaltrials.gov/show/nct00585650 (first received 3 January 2008).
NCT00645892 {published data only}
-
- NCT00645892. Extension study of two dosing schedules of adalimumab in subjects with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct00645892 (first received 28 March 2008).
NCT00646191 {published data only}
-
- NCT00646191. Study of the safety and efficacy of adalimumab in subjects with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct00646191 (first received 28 March 2008).
NCT00647400 {published data only}
-
- NCT00647400. Adalimumab in adult Japanese subjects with psoriasis. clinicaltrials.gov/show/nct00647400 (first received 31 March 2008).
NCT00832364 {published data only}
-
- NCT00832364. Trial of an injectable biologic and U0279 as combination therapy for severe plaque-type psoriasis. clinicaltrials.gov/show/nct00832364 (first received 30 January 2009).
NCT01163253 {published data only}
-
- NCT01163253. A long term study to evaluate the safety and tolerability of CP-690,550 for patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/nct01163253 (first received 15 July 2010).
NCT01235442 {published data only}
-
- NCT01235442. Evaluate efficacy, and safety of topical therapy and etanercept in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01235442 (first received 5 November 2010).
NCT01276847 {published data only}
-
- NCT01276847. A study to assess the effect of ustekinumab (Stelara®) and etanercept (Enbrel®) in participants with moderate to severe psoriasis (MK-0000-206). clinicaltrials.gov/show/nct01276847 (first received 13 January 2011).
NCT01412944 {published data only}
-
- NCT01412944. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE). clinicaltrials.gov/show/nct01412944 (first received 9 August 2011).
NCT01443338 {published data only}
-
- NCT01443338. Study evaluating the efficacy and safety of triptergium wilfordii and acitretin in psoriasis vulgaris - CHINA201002016-2. clinicaltrials.gov/show/nct01443338 (first received 29 September 2011).
NCT01544595 {published data only}
-
- NCT01544595. Extension study of secukinumab prefilled syringes in subjects with moderate to severe chronic plaque-type psoriasis completing preceding psoriasis phase III studies with secukinumab. clinicaltrials.gov/show/nct01544595 (first received 6 March 2012).
NCT01550744 {published data only}
-
- NCT01550744. A study of ustekinumab to evaluate a "subject-tailored" maintenance dosing approach in subjects with moderate-to-severe plaque psoriasis (PSTELLAR). clinicaltrials.gov/show/nct01550744 (first received 12 March 2012).
NCT01624233 {published data only}
-
- NCT01624233. A study in Japanese participants with moderate-to-severe psoriasis (UNCOVER-J). clinicaltrials.gov/show/nct01624233 (first received 20 June 2012).
NCT01722214 {published data only}
-
- NCT01722214. Trial on the effect of adalimumab on vascular inflammation in patients with psoriasis. clinicaltrials.gov/show/nct01722214 (first received 6 November 2012).
NCT01806597 {published data only}
-
- NCT01806597. Study of safety, tolerability, and efficacy of secukinumab in subjects with moderate to severe palmoplantar psoriasis (GESTURE). clinicaltrials.gov/show/nct01806597 (first received 7 March 2013).
NCT01815723 {published data only}
-
- NCT01815723. Efficacy study on dimethyl fumarate to treat moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01815723 (first received 21 March 2013).
NCT01828086 {published data only}
-
- Kaul M, Jarvis P, Rozenberg I, Kolbinger F, Di Padova F, Calonder C, et al. First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2021;35(5):1143-51. - PMC - PubMed
-
- NCT01828086. Single and multiple dose escalation study to assess the safety and tolerability of CJM112 in psoriasis. clinicaltrials.gov/show/nct01828086 (first received 10 April 2013).
NCT01936688 {published data only}
-
- EUCTR2013-001740-54-HU. A clinical research study of 28 weeks to test the safety/tolerability and effectiveness of an investigational study medication (subcutaneous SCH 900222/MK-3222) in improving the signs and symptoms of moderate-to-severe chronic plaque psoriasis, and to compare it to an approved medication for the treatment of psoriasis called etanercept. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-001740-54-HU (first received 18 September 2013).
-
- NCT01936688. A study to evaluate the efficacy and safety/tolerability of subcutaneous MK-3222 in participants with moderate-to-severe chronic plaque psoriasis (MK-3222-012). clinicaltrials.gov/show/nct01936688 (first received 6 September 2013).
NCT02362789 {published data only}
-
- NCT02362789. Secukinumab study in PSOriasis exploring pruRITUS intensity and lesional biomarkers (PSORITUS). clinicaltrials.gov/show/nct02362789 (first received 13 February 2015).
NCT02409667 {published data only}
-
- NCT02409667. Plaque psoriasis efficacy and safety with secukinumab (OPTIMISE). clinicaltrials.gov/show/nct02409667 (first received 7 April 2015).
NCT02798211 {published data only}
-
- NCT02798211. Study to evaluate the safety and efficacy of secukinumab 300 mg and 150 mg in adult patients with active psoriatic arthritis (PsA) after 16 weeks of treatment compared to placebo. clinicaltrials.gov/show/nct02798211 (first received 14 June 2016).
NCT03010527 {published data only}
-
- NCT03010527. Study to evaluate the long-term safety, tolerability and efficacy of bimekizumab in patients with chronic plaque psoriasis. clinicaltrials.gov/show/nct03010527 (first received 5 January 2017).
NCT03020199 {published data only}
-
- EUCTR2015-002423-26-FI. Study of the efficacy of early intervention with secukinumab 300 mg s.c. compared to narrow-band UVB in patients with new-onset moderate to severe plaque psoriasis. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-002423-26-FI (first received 21 October 2016).
-
- NCT03020199. Study of the efficacy of early intervention with secukinumab 300 mg s.c. compared to narrow-band UVB in patients with new-onset moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct03020199 (first received 13 January 2017).
NCT03025542 {published data only}
-
- NCT03025542. Study to evaluate the pharmacokinetics (PK), pharmacodynamics (PD), and safety of bimekizumab in patients with chronic plaque psoriasis. clinicaltrials.gov/show/nct03025542 (first received 19 January 2017).
NCT03073213 {published data only}
-
- NCT03073213. A study of ixekizumab in Chinese participants with psoriasis vulgaris. clinicaltrials.gov/show/nct03073213 (first received 8 March 2017).
NCT03210259 {published data only}
-
- NCT03210259. The VOLTAIRE-X trial looks at the effect of switching between Humira® and BI 695501 in patients with plaque psoriasis. clinicaltrials.gov/show/nct03210259 (first received 6 July 2017).
NCT03482011 {published data only}
-
- Anonymous. Efficacy, safety, and quality of life in patients with moderate-to-severe plaque psoriasis treated with mirikizumab (LY3074828) in a phase 2 study. Journal of the American Academy of Dermatology 2018;79(3 Suppl 1):AB126.
-
- NCT03482011. A study to evaluate the efficacy and safety of mirikizumab (LY3074828) in participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/nct03482011 (first received 29 March 2018).
NCT03598790 {published data only}
-
- NCT03598790. A study to assess the safety, tolerability and efficacy of bimekizumab in adult subjects with moderate to severe chronic plaque psoriasis (BE BRIGHT). clinicaltrials.gov/show/nct03598790 (first received 25 July 2018).
NCT04121143 {published data only}
-
- NCT04121143. A phase II clinical study of SHR-1314 injection in the treatment of moderate to severe plaque psoriasis in adults. clinicaltrials.gov/show/NCT04121143 (first received 9 October 2019).
NCT04488185 {published data only}
-
- NCT04488185. An efficacy study of secukinumab in plaque psoriasis patients with subclinical psoriatic arthritis as measured by musculoskeletal ultrasound (INTERCEPT). clinicaltrials.gov/show/NCT04488185 (first received 27 July 2020).
NCT04614298 {published data only}
-
- NCT04614298. A phase 4 study of brodalumab (KHK4827) in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04614298 (first received 3 November 2020).
NCT04839016 {published data only}
-
- NCT04839016. Efficacy, safety and PK of SHR-1314 in patients with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/NCT04839016 (first received 9 April 2021).
NCT04882098 {published data only}
-
- NCT04882098. A study of guselkumab in participants with active psoriatic arthritis. clinicaltrials.gov/show/NCT04882098 (first received 11 May 2021).
NCT05073315 {published data only}
-
- NCT05073315. A comparative study between ABP 501 and Humira® in participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT05073315 (first received 11 October 2021).
NCT05184348 {published data only}
-
- NCT05184348. Plexin B2 gene expression and polymorphisms in psoriasis: relation to narrow band ultraviolet B, acitretin and combined therapy. clinicaltrials.gov/show/NCT05184348 (first received 11 January 2022).
NCT05478499 {published data only}
-
- NCT05478499. Efficacy and safety of deucravacitinib versus placebo in participants with moderate-to-severe scalp psoriasis. clinicaltrials.gov/show/NCT05478499 (first received 28 July 2022).
NCT05495568 {published data only}
-
- NCT05495568. To compare pharmacokinetics, efficacy, and safety of CT-P17 with Humira in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT05495568 (first received 10 August 2022).
Nemoto 2018 {published data only}
-
- Nemoto O, Hirose K, Shibata S, Li K, Kubo H. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. British Journal of Dermatology 2018;178(3):689-96. [CENTRAL: CN-01449577] - PubMed
Nieboer 1990 {published data only}
-
- Nieboer C, De Hoop D, Langendijk PN, Van Loenen AC, Gubbels J. Fumaric acid therapy in psoriasis: a double-blind comparison between fumaric acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatologica 1990;181(1):33-7. [CENTRAL: CN-00351435] - PubMed
Nijsten 2008 {published data only}
-
- Nijsten T, Spuls P, Stern RS. STROBE: a beacon for observational studies. Archives of Dermatology 2008;144(9):1200-4. [PMID: ] - PubMed
Noda 2011 {published data only}
-
- Noda S, Mizuno K, Adachi M. Treatment effect of adalimumab and infliximab in Japanese psoriasis patients: results in a single community-based hospital. Journal of Investigative Dermatology 2011;131(Suppl 2):S38. [EMBASE: 70520994] - PubMed
Noor 2017 {published data only}
-
- Noor SM, Ayub N, Paracha MM. Efficacy and safety of methotrexate versus acitretin in chronic plaque psoriasis. Journal of Postgraduate Medical Institute 2017;31(1):4-7. [CENTRAL: CN-01340758]
Novotny 1973 {published data only}
-
- Novotny F. Use of methotrexate in psoriasis. Ceskoslovenska Dermatologie 1973;48(5):301-5. [PMID: ] - PubMed
Nyfors 1978 {published data only}
-
- Nyfors A. Benefits and adverse drug experiences during long-term methotrexate treatment of 248 psoriatics. Danish Medical Bulletin 1978;25(5):208-11. [PMID: ] - PubMed
Okubo 2019 {published data only}
-
- Okubo Y, Ohtsuki M, Morita A, Yamaguchi M, Shima T, Tani Y, et al. Long-term efficacy and safety of secukinumab in Japanese patients with moderate to severe plaque psoriasis: 3-year results of a double-blind extension study. Journal of Dermatology 2019;46(3):186-92. [CENTRAL: CN-01794517] - PMC - PubMed
Oliver 2021 {published data only}
-
- Oliver R, Krueger JG, Glatt S, Vajjah P, Mistry C, Page M, et al. Bimekizumab for the treatment of moderate to severe plaque psoriasis: efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase 2a randomized, multicenter double-blinded study. British journal of dermatology 2021;186(4):652-63. - PMC - PubMed
OPT Pivotal‐1 2015 {published data only}
-
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. British Journal of Dermatology 2015;173(4):949-61. [CENTRAL: CN-01105187] [PMID: ] - PubMed
OPT Pivotal‐2 2015 {published data only}
-
- Papp KA, Menter MA, Abe M, Elewski B, Feldman SR, Gottlieb AB, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. British Journal of Dermatology 2015;173(4):949-61. [CENTRAL: CN-01105187] [PMID: ] - PubMed
Orfanos 1978 {published data only}
-
- Orfanos CE, Goerz G. Oral psoriasis treatment with a new aromatic retinoid (Ro 10-9359): a multi-centre controlled study of 291 patients (preliminary results). Deutsche Medizinische Wochenschrift 1978;103(5):195-9. [CENTRAL: CN-00017768] - PubMed
Orfanos 1979 {published data only}
-
- Orfanos CE, Steigleder GK, Pullmann H, Bloch PH. Oral retinoid and UVB radiation: a new, alternative treatment for psoriasis on an out-patient basis. Acta Dermato-Venereologica 1979;59(3):241-4. [CENTRAL: CN-00020394] - PubMed
Ortonne 2008 {published data only}
-
- Ortonne JP, Griffiths CE, Dauden E, Strohal R, Robertson D, Pedersen R, et al. Efficacy and safety of continuous versus paused etanercept treatment in patients with moderate-to-severe psoriasis over 54 weeks: the CRYSTEL Study. Expert Review of Dermatology 2008;3(6):657-65. [CENTRAL: CN-00754922]
Ortonne 2011 {published data only}
-
- Ortonne JP, Chimenti S, Reich K, Gniadecki R, Sprøgel P, Unnebrink K, et al. Efficacy and safety of adalimumab in patients with psoriasis previously treated with anti-tumour necrosis factor agents: subanalysis of BELIEVE. Journal of the European Academy of Dermatology and Venereology 2011;25(9):1012-20. [CENTRAL: CN-00812830] - PubMed
Osamu 2014 {published data only}
-
- Osamu N, Hirotaka N, Koji S, Kenji T. Clinical pharmacology of the anti-IL-17 receptor antibody brodalumab (KHK4827) in Japanese normal healthy volunteers and Japanese subjects with moderate to severe psoriasis: a randomized, dose-escalation, placebo-controlled study. Journal of Dermatological Science 2014;75(3):201-4. [CENTRAL: CN-00999213] - PubMed
Page 2020 {published data only}
-
- Page KM, Suarez-Farinas M, Suprun M, Zhang W, Garcet S, Fuentes-Duculan J, et al. Molecular and cellular responses to the TYK2/JAK1 inhibitor PF-06700841 reveal reduction of skin inflammation in plaque psoriasis. Journal of Investigative Dermatology 2020;140(8):1546-55.e4. - PubMed
Pakozdi 2018 {published data only}
-
- Pakozdi T, Georgantas RW, Grebe KM, Visvanathan S, Baum P, Davis W. RNA-seq genomic analysis demonstrated the molecular efficacy of risankizumab in a moderate-to-severe plaque psoriasis phase 2 clinical study. Experimental Dermatology 2018;27(Suppl 2):21. [CENTRAL: CN-01793116]
Papp 2001 {published data only}
-
- Papp K, Bissonnette R, Krueger JG, Carey W, Gratton D, Gulliver WP, et al. The treatment of moderate to severe psoriasis with a new anti-CD11a monoclonal antibody. Journal of the American Academy of Dermatology 2001;45(5):665-74. [CENTRAL: CN-00374574] - PubMed
Papp 2006 {published data only}
-
- Papp K, Langley R, Bissonnette R, Rosoph L. A phase III, randomized, multicenter, double-blind, placebo-controlled study of ISA247 in plaque psoriasis patients. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB9. [CENTRAL: CN-00602194] - PubMed
Papp 2008 {published data only}
-
- Papp K, Bissonnette R, Rosoph L, Wasel N, Lynde CW, Searles G, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet 2008;371(9521):1337-42. [CENTRAL: CN-00631348] - PubMed
Papp 2009 {published data only}
-
- Papp K, Okun M, Vender R. Adalimumab in the treatment of psoriasis: pooled efficacy and safety results from three pivotal studies. Journal of Cutaneous Medicine and Surgery 2009;13(Suppl 2):S58-66. [PMID: ] - PubMed
Papp 2011a {published data only}
-
- Papp K, Signorovitch J, Mulani P, Bao Y. Comparison of psoriasis sign and symptom reduction and complete clearance with adalimumab versus etanercept. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB153. [CENTRAL: CN-00843873]
Papp 2011b {published data only}
-
- Papp K, Signorovitch J, Sundaram M, Bao Y. Effects of abt-874 treatment on health-related quality of life and work productivity and activity impairment in patients with psoriasis. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB155. [CENTRAL: CN-00843874]
Papp 2011c {published data only}
-
- Papp K, Yu A, Sundaram M, Bao Y. Achieving long-term sustained response is associated with improvements in patient-reported outcomes in patients with psoriasis treated with ABT-874. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB160. [CENTRAL: CN-00843875]
Papp 2012b {published data only}
-
- Bushmakin AG, Mamolo C, Cappelleri JC, Stewart M. The relationship between pruritus and the clinical signs of psoriasis in patients receiving tofacitinib. Journal of Dermatological Treatment 2015;26(1):19-22. [CENTRAL: CN-01051702] [PMID: ] - PubMed
-
- Mamolo C, Harness J, Tan H, Menter A. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis. Journal of the European Academy of Dermatology and Venereology 2014;28(2):192-203. [CENTRAL: CN-00959043] [PMID: ] - PubMed
-
- Mamolo CM, Bushmakin AG, Cappelleri JC. Application of the Itch Severity Score in patients with moderate-to-severe plaque psoriasis: clinically important difference and responder analyses. Journal of Dermatological Treatment 2015;26(2):121-3. [CENTRAL: CN-01083900] [PMID: ] - PubMed
-
- Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. British Journal of Dermatology 2012;167(3):668-77. [CENTRAL: CN-00856433] [PMID: ] - PubMed
-
- Strober B, Buonanno M, Clark JD, Kawabata T, Tan H, Wolk R, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. British Journal of Dermatology 2013;169(5):992-9. [CENTRAL: CN-01122547] [PMID: ] - PubMed
Papp 2012d {published data only}
-
- Papp KA, Reid C, Foley P, Sinclair R, Salinger DH, Williams G, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. Journal of Investigative Dermatology 2012;132(10):2466-9. [CENTRAL: CN-00854479] - PubMed
Papp 2012e {published data only}
-
- Papp KA, Poulin Y, Bissonnette R, Bourcier M, Toth D, Rosoph L, et al. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. Journal of the American Academy of Dermatology 2012;66(2):e33-45. [CENTRAL: CN-00883092] - PubMed
Papp 2017c {published data only}
-
- Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. British Journal of Dermatology 2017;177(6):1562-74. [CENTRAL: CN-01440056] - PubMed
Papp 2018a {published data only}
-
- Papp K, Kimball A, Blauvelt A, Reich K, Gooderham M, Tyring S, et al. Effect of tildrakizumab on personal relationships in patients with moderate-to-severe chronic plaque psoriasis. Acta Dermato-Venereologica 2018;98(Suppl 219):54. [CENTRAL: CN-01620176]
Papp 2018b {published data only}
-
- Papp KA, Blauvelt A, Kimball AB, Han C, Randazzo B, Wasfi Y, et al. Patient-reported symptoms and signs of moderate-to-severe psoriasis treated with guselkumab or adalimumab: results from the randomized VOYAGE 1 trial. Journal of the European Academy of Dermatology and Venereology 2018;32(9):1515-22. [CENTRAL: CN-01665289] [DOI: 10.1111/jdv.14910] - DOI - PMC - PubMed
Park 2013 {published data only}
-
- Park KK, Wu JJ, Koo J. A randomized, 'head-to-head' pilot study comparing the effects of etanercept monotherapy vs. etanercept and narrowband ultraviolet B (NB-UVB) phototherapy in obese psoriasis patients. Journal of the European Academy of Dermatology and Venereology 2013;27(7):899-906. [CENTRAL: CN-00969013] - PubMed
Paul 2012 {published data only}
-
- Paul C, Van de Kerkhof P, Puig L, Unnebrink K, Goldblum O, Thaçi D. Influence of psoriatic arthritis on the efficacy of adalimumab and on the treatment response of other markers of psoriasis burden: subanalysis of the BELIEVE study. European Journal of Dermatology 2012;22(6):762-9. [CENTRAL: CN-00966715] - PubMed
Paul 2014 {published data only}
-
- Paul C, Puig L, Kragballe K, Luger T, Lambert J, Chimenti S, et al. Transition to ustekinumab in patients with moderate-to-severe psoriasis and inadequate response to methotrexate: a randomized clinical trial (TRANSIT). British Journal of Dermatology 2014;170(2):425-34. [CENTRAL: CN-00982375] - PubMed
Paul 2018 {published data only}
-
- Paul C, Guenther L, Torii H, Sofen H, Burge R, Lin CY, et al. Impact of ixekizumab on facial psoriasis and related quality of life measures in moderate-to-severe psoriasis patients: 12-week results from two phase III trials. Journal of the European Academy of Dermatology and Venereology 2018;32(1):68-72. [CENTRAL: CN-01449415] [DOI: 10.1111/jdv.14581] - DOI - PubMed
Perks 2017 {published data only}
-
- Perks B. Randomized non-inferiority trial fails to find inferiority switching from infliximab originator to CT-P13 biosimilar. GaBI Journal 2017;6(4):188-9. [DOI: 10.5639/gabij.2017.0604.042] - DOI
Pettit 1979 {published data only}
-
- Pettit JH. Oral retinoid for psoriasis. A report of a double blind study. Acta Dermato-Venereologica 1979;59(85 Suppl):133-6. [CENTRAL: CN-00021931] [MEDLINE: ] - PubMed
Petzelbauer 1990 {published data only}
-
- Petzelbauer P, Honigsmann H, Langer K, Anegg B, Strohal R, Tanew A, et al. Cyclosporin A in combination with photochemotherapy (PUVA) in the treatment of psoriasis. British Journal of Dermatology 1990;123(5):641-7. [CENTRAL: CN-00351600] - PubMed
Piascik 2003 {published data only}
-
- Piascik P. Alefacept, first biologic agent approved for treatment of psoriasis. Journal of the American Pharmacists Association 2003;43(5):649-50. [PMID: ] - PubMed
Ports 2013 {published data only}
Puig 2018 {published data only}
-
- Puig L, Augustin M, Blauvelt A, Gottlieb AB, Vender R, Korman NJ, et al. Effect of secukinumab on quality of life and psoriasis-related symptoms: a comparative analysis versus ustekinumab from the CLEAR 52-week study. Journal of the American Academy of Dermatology 2018;78(4):741-8. [CENTRAL: CN-01463902] - PubMed
Punwani 2012 {published data only}
-
- Punwani N, Scherle P, Flores R, Shi J, Liang J, Yeleswaram S, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. Journal of the American Academy of Dermatology 2012;67(4):658-64. [CENTRAL: CN-00881387] - PubMed
Rabasseda 2012 {published data only}
-
- Rabasseda X. A report from the American Academy of Dermatology 70th Annual Meeting (March 16-20, 2012; San Diego, California, USA). Drugs of Today 2012;48(5):367-73. [PMID: ] - PubMed
Radmanesh 2011 {published data only}
-
- Radmanesh M, Rafiei B, Moosavi ZB, Sina N. Weekly vs. daily administration of oral methotrexate (MTX) for generalized plaque psoriasis: a randomized controlled clinical trial. International Journal of Dermatology 2011;50(10):1291-3. [CENTRAL: CN-00805615] - PubMed
Raman 1998 {published data only}
-
- Raman GV, Campbell SK, Farrer A, Albano JD, Cook J. Modifying effects of amlodipine on cyclosporin A-induced changes in renal function in patients with psoriasis. Journal of Hypertension 1998;16(4 Suppl):S39-41. [CENTRAL: CN-00308573] - PubMed
Reich 2004 {published data only}
-
- Reich K. Alefacept in the treatment of psoriasis for whom conventional therapies are ineffective or inappropriate. Journal of the European Academy of Dermatology and Venereology 2004;18(6):808. [CENTRAL: CN-00550795]
-
- Sclessinger J, Pariser R, Park S, Wierz G. Evaluation of the efficacy and safety of alefacept in patients for whom conventional psoriasis therapies are ineffective or inappropriate. Journal of the American Academy of Dermatology 2007;56(2):AB192. [CENTRAL: CN-00616055]
Reich 2011 {published data only}
-
- Reich K, Van Hoogstraten BJ, Wozel G, Zheng H, Flint L. Long-term efficacy and safety of maintenance versus intermittent infliximab therapy for moderate to severe plaque-type psoriasis: the Restore2 trial. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB150. [CENTRAL: CN-00843879]
Reich 2014 {published data only}
-
- Reich K, Puig L, Paul C, Kragballe K, Luger T, Lambert J, et al. One-year safety and efficacy of ustekinumab and results of dose adjustment after switching from inadequate methotrexate treatment: the TRANSIT randomized trial in moderate-to-severe plaque psoriasis. British Journal of Dermatology 2014;170(2):435-44. [CENTRAL: CN-00982376] - PubMed
Reich 2016a {published data only}
-
- Reich K, Soung J, Gooderham M, Zhang Z, Nograles K, Goodfield M. LIBERATE trial: sustained efficacy of apremilast in patients with moderate-to-severe psoriasis who continued on apremilast or switched from etanercept treatment. British Journal of Dermatology 2016;175(S1):71-2. [CENTRAL: CN-01303307] [DOI: 10.1111/bjd.14524] - DOI
Reich 2016b {published data only}
-
- Reich K, Choi SL, Jackson K, Mallbris L, Blauvelt A. Time course of ixekizumab drug levels and the relationship at week 60 to efficacy in patients with moderate-to- severe plaque psoriasis (UNCOVER-3). Experimental Dermatology 2016;25(S4):39. [CENTRAL: CN-01407586]
Reich 2017a {published data only}
-
- Reich K, Goodfield M, Green L, Nograles K, Chen R, Levi E, et al. Efficacy and safety of apremilast through 104 weeks in subjects with moderate to severe psoriasis randomized to placebo, apremilast, or etanercept who continued on or switched to apremilast after week 16 in a phase 3B study. Arthritis and Rheumatology 2017;69(Suppl 10):627.
Reich 2017b {published data only}
-
- Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. Journal of the American Academy of Dermatology 2017;76(3):418-31. [CENTRAL: CN-01341399] - PubMed
Reich 2017c {published data only}
Reich 2018a {published data only}
-
- Reich K, Sullivan J, Arenberger P, Mrowietz U, Jazayeri S, Augustin M, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. British Journal of Dermatology 2018 Oct 26 [Epub ahead of print]. [DOI: 10.1111/bjd.17351] - DOI - PubMed
Reich 2018b {published data only}
-
- Reich K, Jackson K, Ball S, Garces S, Kerr L, Chua L, et al. Ixekizumab pharmacokinetics, anti-drug antibodies, and efficacy through 60 weeks of treatment of moderate to severe plaque psoriasis. Journal of Investigative Dermatology 2018;138(10):2168-73. [CENTRAL: CN-01611449] - PubMed
Reich 2018c {published data only}
-
- Reich K, Gooderham M, Thaçi D, Crowley JJ, Ryan C, Krueger JG, et al. Efficacy and safety of risankizumab (RZB) compared with adalimumab (ADA) in patients with moderate-to-severe plaque psoriasis: results from the phase 3 IMMvent trial. Experimental Dermatology 2018;27(Suppl 2):9-10. [CENTRAL: CN-01790123]
Reich 2019 {published data only}
-
- Bissonnette R, Maari C, Menter MA, Ohata C, Papp KA, Tuttle JL, et al. 15328 Efficacy and safety of mirikizumab in patients with moderate to severe plaque psoriasis: 104-week results from a randomized phase 2 study. Journal of the American Academy of Dermatology 2020;83(6 Suppl):AB147.
-
- Gooderham M, Edson-Heredia E, Li J, Patel D, Guo J, Bagel J. Patient-reported improvements in health-related quality of life by improvements in clinician-rated psoriasis severity: a phase 2 study analysis in patients with psoriasis treated with mirikizumab. Journal of the Dermatology Nurses' Association 2020;12(2):No-pagination.
-
- NCT02899988. A study of mirikizumab (LY3074828) in participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02899988 (first received 14 September 2016).
-
- Papp K, Maari C, Rich P, Klekotka P, Li J, Tuttle J, et al. Response to mirikizumab at week 52 among patients who did not achieve a PASI 90 response at week 16. Journal of Clinical and Aesthetic Dermatology 2019;12(5 Suppl 1):S29.
-
- Reich K, Bissonnette R, Menter A, Klekotka P, Patel D, Li J, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a phase II study. British Journal of Dermatology 2017;177(5):e249. - PubMed
Reitamo 1999 {published data only}
-
- Reitamo S, Spuls P, Sassolas B, Lahfa M, Claudy A, Griffiths C, et al. A double-blind study in patients with severe psoriasis to assess the clinical activity and safety of rapamycin (sirolimus) alone or in association with a reduced dose of cyclosporine. British Journal of Dermatology 1999;141(5):978-9. [CENTRAL: CN-00428747]
Reitamo 2001 {published data only}
-
- Reitamo S, Spuls P, Sassolas B, Lahfa M, Claudy A, Griffiths CE, et al. Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: a randomized controlled trial. British Journal of Dermatology 2001;145(3):438-45. [CENTRAL: CN-00356242] - PubMed
Rim 2003 {published data only}
-
- Rim JH, Park JY, Choe YB, Youn JI. The efficacy of calcipotriol + acitretin combination therapy for psoriasis: comparison with acitretin monotherapy. American Journal of Clinical Dermatology 2003;4(7):507-10. [CENTRAL: CN-00450180] - PubMed
Rinsho Iyaku 1991 {published data only}
-
- Clinical Study Group for Ciclosporin. Clinical efficacy of ciclosporin in the treatment of psoriasis: multicenter double blind study. Rincho Iyaku (Journal of Clinical Therapeutics and Medicines) 1991;7(3):617-33. [CENTRAL: CN-00545330]
Ritchlin 2006a {published data only}
-
- Ritchlin CT. The efficacy and safety of adalimumab in psoriatic arthritis. Current Rheumatology Reports 2006;8(5):329. [CENTRAL: CN-00898612] - PubMed
Ritchlin 2006b {published data only}
-
- Ritchlin CT. The efficacy and safety of alefacept in psoriatic arthritis. Current Rheumatology Reports 2006;8(5):330-1. [CENTRAL: CN-00898611] - PubMed
Romiti 2017 {published data only}
-
- Romiti R, Papadimitropoulos M, Lin C, Burge RT, Zhu B, Garcia EG. Ixekizumab treatment improves itching and health-related quality (HRQOL) of life in psoriasis patients in Latin America. Value in Health 2017;20(9):A807. [CENTRAL: CN-01431329]
RPCEC00000201 {unpublished data only}
-
- RPCEC00000201. Itolizumab for moderate-to-severe psoriasis-phase 3 [Randomized controlled double blind trial to study safety and efficacy of itolizumab (antiCD6) in moderate-to-severe psoriasis]. registroclinico.sld.cu/trials/RPCEC00000201-En (first received 15 October 2015). [CENTRAL: CN-01835365]
Ryan 2018 {published data only}
-
- Ryan C, Menter A, Guenther L, Blauvelt A, Bissonnette R, Meeuwis K, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. British Journal of Dermatology 2018;179(4):844-52. [CENTRAL: CN-01630323] - PubMed
Saeki 2017 {published data only}
-
- Saeki H, Nakagawa H, Nakajo K, Ishii T, Morisaki Y, Aoki T, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). Journal of Dermatology 2017;44(4):355-62. [DOI: 10.1111/1346-8138.13622] [PMID: ] - DOI - PMC - PubMed
Salim 2006 {published data only}
-
- Salim A, Tan E, Ilchyshyn A, Berth-Jones J. Folic acid supplementation during treatment of psoriasis with methotrexate: a randomized, double-blind, placebo-controlled trial. British Journal of Dermatology 2006;154(6):1169-74. [CENTRAL: CN-00565411] - PubMed
Scholl 1981 {published data only}
-
- Scholl E. Treatment of psoriasis on an outpatient-base using UVB-radiations, oral retinoid and ten percent saline baths. Schweizerische Rundschau für Medizin Praxis 1981;70(41):1806-16. [CENTRAL: CN-00026632] - PubMed
Schopf 1998 {published data only}
-
- Schopf RE, Hultsch T, Lotz J, Brautigam M. Eosinophils, pruritus and psoriasis: effects of treatment with etretinate or cyclosporin-A. Journal of the European Academy of Dermatology and Venereology 1998;11(3):234-9. [CENTRAL: CN-00158727] [PMID: ] - PubMed
Schulze 1991 {published data only}
-
- Schulze HJ. Comparative trial of sandimmune and etretinate for plaque-type psoriasis. Zeitschrift fur Hautkrankheiten 1991;66(Suppl 1):33-8. [CENTRAL: CN-00180765]
Shintani 2011 {published data only}
-
- Shintani Y, Kaneko N, Furuhashi T, Saito C, Morita A. Safety and efficacy of a fixed-dose cyclosporin microemulsion (100 mg) for the treatment of psoriasis. Journal of Dermatology 2011;38(10):966-72. [CENTRAL: CN-00811861] - PubMed
Shiohara 1992 {published data only}
-
- Shiohara T, Imanishi K, Sagawa Y, Nagashima M. Differential effects of cyclosporine and etretinate on serum cytokine levels in patients with psoriasis. Journal of the American Academy of Dermatology 1992;27(4):568-74. [CENTRAL: CN-00361111] - PubMed
Shupack 1997 {published data only}
-
- Shupack J, Abel E, Bauer E, Brown M, Drake L, Freinkel R, et al. Cyclosporine as maintenance therapy in patients with severe psoriasis. Journal of the American Academy of Dermatology 1997;36(3 pt 1):423-32. [CENTRAL: CN-00137758] - PubMed
Simonova 2005 {published data only}
-
- Simonova OV, Nemtsov BF. Psoriatic arthritis: combined treatment with prospidin and methotrexate. Terapevticheskii Arkhiv 2005;77(8):60-4. [CENTRAL: CN-00530903] - PubMed
Sinclair 2017 {published data only}
-
- Sinclair R, Reich K, Papp K, Tyring SS, Thaçi D, Cichanowitz N, et al. Tildrakizumab, a selective IL-23p19 antibody, in the treatment of chronic plaque psoriasis: results from two randomised, controlled, phase 3 trials. Australasian Journal of Dermatology 2017;58(S1):9-10. [CENTRAL: CN-01378810] [DOI: 10.1111/ajd.12652] - DOI
Sofen 2011 {published data only}
-
- Sofen H, Smith S, Matheson R, Leonardi C, Calderon C, Bouman-Thio E, et al. Results of a single ascending dose study to assess the safety and tolerability of CNTO 1959 following intravenous or subcutaneous administration in healthy subjects and in subjects with moderate to severe psoriasis. British Journal of Dermatology 2011;165(6):e10. [CENTRAL: CN-01020427]
Sofen 2014 {published data only}
-
- Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. Journal of Allergy and Clinical Immunology 2014;133(4):1032-40. [CENTRAL: CN-00984656] - PubMed
Soung 2022 {published data only}
-
- Soung J, Cather JC, Gooderham M. 33054 Improvement in touch avoidance in patients with genital psoriasis treated with ixekizumab: 52-week results of a phase 3 clinical trial in patients with moderate-to-severe genital psoriasis (IXORA-Q). Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB69.
Spadaro 2008 {published data only}
-
- Spadaro A, Ceccarelli F, Scrivo R, Valesini G. Life-table analysis of etanercept with or without methotrexate in patients with psoriatic arthritis. Annals of the Rheumatic Diseases 2008;67(11):1650-1. [CENTRAL: CN-00651403] - PubMed
Spuls 2012 {published data only}
-
- Spuls PI, Hooft L. Brodalumab and ixekizumab, anti-interleukin-17-receptor antibodies for psoriasis: a critical appraisal. British Journal of Dermatology 2012;167(4):710-3; discussion 714-5. [PMID: ] - PubMed
Stein Gold 2018 {published data only}
-
- Stein Gold L, Bagel J, Lebwohl M, Jackson JM, Chen R, Goncalves J, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. Journal of Drugs in Dermatology 2018;17(2):221-8. [CENTRAL: CN-01627309] - PubMed
Stein Gold 2021 {published data only}
-
- Stein Gold L, Papp K, Pariser D, Green L, Bhatia N, Sofen H, et al. Efficacy and safety of apremilast in patients with mild-to-moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Journal of the American Academy of Dermatology 2021;31:31. - PubMed
Sticherling 1994 {published data only}
-
- Sticherling M. Therapy of severe psoriasis with Sandimmune. Hautarzt (Symposium of Nurnberg Sandoz AG 13 February 1993, Nurnberg) 1994;45(1):50-2. [PMID: ] - PubMed
Strober 2004 {published data only}
-
- Strober BE, Clarke S. Etanercept for the treatment of psoriasis: combination therapy with other modalities. Journal of Drugs in Dermatology 2004;3(3):270-2. [PMID: ] - PubMed
Strober 2012 {published data only}
-
- Strober BE, Sobell JM, Duffin KC, Bao Y, Guerin A, Yang H, et al. Sleep quality and other patient-reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: results from PROGRESS, an open-label phase IIIB trial. British Journal of Dermatology 2012;167(6):1374-81. [PMID: ] - PubMed
Strober 2017a {published data only}
-
- Strober B, Gottlieb AB, Sherif B, Mollon P, Gilloteau I, McLeod L, et al. Secukinumab sustains early patient-reported outcome benefits through 1 year: results from 2 phase III randomized placebo-controlled clinical trials comparing secukinumab with etanercept. Journal of the American Academy of Dermatology 2017;76(4):655-61. [CENTRAL: CN-01368337] [DOI: 10.1016/j.jaad.2016.11.043] - DOI - PubMed
Strober 2017b {published data only}
-
- Strober B, Bagel J, Lebwohl M, Stein Gold L, Jackson JM, Chen R, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. Journal of Drugs in Dermatology 2017;16(8):801-8. [CENTRAL: CN-01416087] - PubMed
Strober 2017c {published data only}
-
- Strober B, Alikhan M, Lockshin B, Schafer P. Cytokine effects of apremilast as a mechanism of efficacy in systemic-naive patients with moderate plaque psoriasis: results from the UNVEIL trial. British Journal of Dermatology 2017;177(5):e256-7. [CENTRAL: CN-01452501] [DOI: 10.1111/bjd.16059] - DOI - PubMed
Strober 2018 {published data only}
-
- Strober B, Forman S, Bagel J, Lebwohl M, Stein Gold L, Jackson JM, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis (52-week results of the UNVEIL study). Journal of Clinical and Aesthetic Dermatology 2018;11(5 Suppl 1):S21-2. [CENTRAL: CN-01713688] - PubMed
Sun 2019 {published data only}
-
- Sun J, Chen G, Wu M, Han Y, Gao H, Zhang T, et al. China-manufactured adalimumab biosimilar, HLX03, demonstrated pharmacokinetic equivalence and comparable safety to adalimumab. Annals of the Rheumatic Diseases 2019;78 Suppl 2:706.
Sweetser 2006 {published data only}
-
- Sweetser M, Ticho B, Swan S. Subcutaneous administration of alefacept is bioequivalent to intramuscular administration: results of a randomized, open-label, crossover study in healthy volunteers. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB224. [CENTRAL: CN-00602513] - PubMed
Syversen 2020 {published data only}
-
- Syversen SW, Goll GL, Jorgensen KK, Olsen IC, Sandanger O, Gehin JE, et al. Therapeutic drug monitoring of infliximab compared to standard clinical treatment with infliximab: study protocol for a randomised, controlled, open, parallel-group, phase IV study (the NOR-DRUM study). Trials 2020;21(1):13. - PMC - PubMed
Talamonti 2021 {published data only}
-
- Talamonti M, Malara G, Natalini Y, Bardazzi F, Conti A, Chiricozzi A, et al. Secukinumab improves anxiety and depression in patients with moderate-to-severe psoriasis: results from the SUPREME study. Australasian Journal of Dermatology 2021;62 Suppl 1:27-8.
Talwar 1992 {published data only}
-
- Talwar S. Methotrexate-puvasol combination in treatment of psoriasis. Indian Journal of Dermatology, Venereology and Leprology 1992;58(1):15-9. [CENTRAL: CN-00663131]
TCTR20190705002 {published data only}
-
- TCTR20190705002. Comparison of the clinical efficacy of subcutaneous versus oral administration of methotrexate in patients with psoriasis vulgaris. www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20190705002 (first received 4 July 2019).
Tejasvi 2012 {published data only}
-
- Tejasvi T, Chow C, Simpson MJ, Ellis CN. Use of clinical trial data to compare psoriasis area and severity index, static physician's global assessment, and lattice system-physician's global assessment in assessing severity of psoriasis. Dermatology and Therapy 2012;2:S55. [CENTRAL: 71025691]
Thaçi 2002 {published data only}
-
- Thaçi D, Bräutigam M, Kaufmann R, Weidinger G, Paul C, Christophers E. Body-weight-independent dosing of cyclosporine micro-emulsion and three times weekly maintenance regimen in severe psoriasis. A randomised study. Dermatology (Basel, Switzerland) 2002;205(4):383-8. [CENTRAL: CN-00411587] - PubMed
Thaçi 2010 {published data only}
-
- Thaçi D, Ortonne JP, Chimenti S, Ghislain PD, Arenberger P, Kragballe K, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. British Journal of Dermatology 2010;163(2):402-11. [CENTRAL: CN-00771848] - PubMed
Thaçi 2018 {published data only}
-
- Thaçi D, Gottlieb AB, Reich K, Bagel J, Peterson L, Purcaru O, et al. Certolizumab pegol improves patient-reported outcomes in chronic plaque psoriasis over 1 year. Acta Dermato-Venereologica 2018;98(Suppl 219):57-8. [CENTRAL: CN-01620168] [DOI: 10.2340/00015555-2978] - DOI
Tong 2008 {published data only}
-
- Tong PZ, Si RL. Effectiveness observation on acitretin capsule for plaque psoriasis. Modern Journal of Integrated Traditional Chinese and Western Medicine [xian Dai Zhong Xi Yi Jie He za Zhi] 2008;17(3):364-5. [CENTRAL: CN-00792764]
Tsakok 2018 {published data only}
-
- Tsakok T, Jabbar-Lopez ZK, Smith CH. Subcutaneous methotrexate in patients with moderate-to-severe psoriasis: a critical appraisal. British Journal of Dermatology 2018;179(1):50-3. [CENTRAL: CN-01742390] - PubMed
Vaclavkova 2014 {published data only}
-
- Chimenti S, Arenberger P, Karpati S, Sator PG, Vaclavkova A, Burcklen M, et al. A phase II study of ponesimod, an oral, selective sphingosine 1-phosphate receptor-1 modulator, in chronic plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2013;27(Suppl 4):22. [CENTRAL: CN-01025267] [PMID: ] - PubMed
-
- Kemeny L, Yankova R, Talamonti M, Vaclavkova A, Burcklen M, Thomas G, et al. A phase II study of ponesimod in chronic plaque psoriasis: improvements in patient-reported outcomes. Journal of the European Academy of Dermatology and Venereology 2013;27(Suppl 4):21. [CENTRAL: CN-01025268] [PMID: ] - PubMed
-
- Vaclavkova A, Chimenti S, Arenberger P, Hollo P, Sator PG, Burcklen M, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014;384(9959):2036-45. [CENTRAL: CN-01040467] [PMID: ] - PubMed
Valenzuela 2017 {published data only}
-
- Valenzuela F, De la Cruz Fernandez C, Galimberti RL, Gurbuz S, McKean-Matthews M, Goncalves L, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis: subgroup analysis of Latin American patients in the phase 3 randomized UNCOVER-3 study. Actas Dermo-Sifiliograficas 2017;108(6):550-63. [CENTRAL: CN-01464996] - PubMed
Van de Kerkhof 2017 {published data only}
-
- Van de Kerkhof P, Guenther L, Gottlieb AB, Sebastian M, Wu JJ, Foley P, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. Journal of the European Academy of Dermatology and Venereology 2017;31(3):477-82. [CENTRAL: CN-01413633] [DOI: 10.1111/jdv.14033] - DOI - PubMed
Van Joost 1988 {published data only}
-
- Van Joost T, Bos JD, Heule F, Meinardi MM. Low-dose cyclosporin A in severe psorasis. A double-blind study. British Journal of Dermatology 1988;118(2):183-90. [CENTRAL: CN-00052789] [PMID: ] - PubMed
Vena 2005 {published data only}
-
- Vena GA, Cassano N, Galluccio A, Loconsole F, Coviello C, Fai D, et al. Evaluation of the efficacy and tolerability of a new intermittent treatment regimen with cyclosporin A in severe psoriasis. Giornale Italiano di Dermatologia e Venereologia 2005;140(5):575-82. [EMBASE: 2006301575]
Vena 2012 {published data only}
-
- Vena GA, Galluccio A, Pezza M, Vestita M, Cassano N. Combined treatment with low-dose cyclosporine and calcipotriol/betamethasone dipropionate ointment for moderate-to-severe plaque psoriasis: a randomized controlled open-label study. Journal of Dermatological Treatment 2012;23(4):255-60. [CENTRAL: CN-00882673] - PubMed
Verma 2021 {published data only}
-
- Verma KK, Kumar P, Bhari N, Gupta S, Kalaivani M. Azathioprine weekly pulse versus methotrexate for the treatment of chronic plaque psoriasis: a randomized controlled trial. Indian Journal of Dermatology, Venereology and Leprology 2021;87(4):509-14. - PubMed
Viglioglia 1978 {published data only}
-
- Viglioglia PA, Barclay A. Oral retinoids and psoriasis. Dermatologica 1978;157(Suppl 1):32-7. [PMID: ] - PubMed
Viswanath 2022 {published data only}
-
- Viswanath V, Joshi P, Lawate P, Tare D, Dhoot D, Mahadkar N, et al. An open-label, randomized, prospective, comparative, three-arm clinical trial to evaluate the safety and effectiveness of apremilast with three different titration methods in patients with chronic plaque psoriasis in India. Psoriasis: Targets and Therapy 2022;12:53-61. - PMC - PubMed
Witkamp 1995 {published data only}
-
- Witkamp L, Zonneveld IM, Jung EG, Schopf RE, Christophers E, Grossman R, et al. Efficacy and tolerability of multiple-dose SDZ IMM 125 in patients with severe psoriasis. British Journal of Dermatology 1995;133(1):95-103. [CENTRAL: CN-00118050] - PubMed
Wolf 2012 {published data only}
-
- Wolf P, Weger W, Legat FJ, Posch-Fabian T, Gruber-Wackernagel A, Inzinger M, et al. Treatment with 311-nm ultraviolet B enhanced response of psoriatic lesions in ustekinumab-treated patients: a randomized intraindividual trial. British Journal of Dermatology 2012;166(1):147-53. [CENTRAL: CN-00841290] - PubMed
Wright 1966 {published data only}
-
- Wright ET, Wolborsky M, Hamer EE. Human low-dosage parenteral methotrexate therapy. A controlled toxicity study. Archives of Dermatology 1966;93(6):731-6. [PMID: ] - PubMed
Wu 2015 {published data only}
Yan 2011 {published data only}
-
- Yan H, Tang M, You Y, Yu JB, Zhang JA, Li XH, et al. Treatment of psoriasis with recombinant human LFA3-antibody fusion protein: a multi-center, randomized, double-blind trial in a Chinese population. European Journal of Dermatology 2011;21(5):737-43. [CENTRAL: CN-00810848] [PMID: ] - PubMed
Yesudian 2013 {published data only}
-
- Yesudian PD, Hashim N, Bharati A, Alkali A, Warren RB, Cox T, et al. A prospective, double-blind, randomized controlled trial of folic acid supplementation vs. placebo in patients with chronic plaque psoriasis treated with methotrexate and effects on serum homocysteine. British Journal of Dermatology 2013;169(Suppl 1):59. [CENTRAL: CN-00873113]
Yiu 2020 {published data only}
-
- Yiu ZZN. Repurposing existing trial data to infer relative efficacy of biologics: guselkumab vs. ustekinumab for psoriasis. British Journal of Dermatology 2020;183(2):202-3. - PubMed
Yoon 2007 {published data only}
-
- Yoon HS, Youn JI. A comparison of two cyclosporine dosage regimens for the treatment of severe psoriasis. Journal of Dermatological Treatment 2007;18(5):286-90. [CENTRAL: CN-00619337] - PubMed
Yosipovitch 2018 {published data only}
-
- Yosipovitch G, Foley P, Ryan C, Cather JC, Meeuwis KA, Burge R, et al. Ixekizumab improved patient-reported genital psoriasis symptoms and impact of symptoms on sexual activity vs placebo in a randomized, double-blind study. Journal of Sexual Medicine 2018;15(11):1645-52. [CENTRAL: CN-01667932] - PubMed
Zachariae 2008 {published data only}
-
- Zachariae C, Mork NJ, Reunala T, Lorentzen H, Falk E, Karvonen SL, et al. The combination of etanercept and methotrexate increases the effectiveness of treatment in active psoriasis despite inadequate effect of methotrexate therapy. Acta Dermato-Venereologica 2008;88(5):495-501. [CENTRAL: CN-00669633] - PubMed
Zhang 2007 {published data only}
-
- Zhang M, Zhang Y-Z, Wang S. The effect of acitretin on moderate to severe plaque psoriasis. Journal of Clinical Dermatology 2007;36(9):592-3. [CENTRAL: CN-00642111]
Zhang 2009a {published data only}
-
- Zhang GL, Huang F, Zhang JL, Li XF. A clinical study of leflunomide and methotrexate therapy in psoriatic arthritis. Chung-Hua Nei Ko Tsa Chih (Chinese Journal of Internal Medicine) 2009;48(7):570-4. [CENTRAL: CN-00732533] - PubMed
Zhang 2009b {published data only}
-
- Zhang LX, Bai YP, Song PH, You LP, Yang DQ. Effect of Chinese herbal medicine combined with acitretin capsule in treating psoriasis of blood-heat syndrome type. Chinese Journal of Integrative Medicine 2009;15(2):141-4. [CENTRAL: CN-00700202] - PubMed
Zhang 2017 {published data only}
-
- NCT01815424. A study evaluating the efficacy and safety of CP-690,550 in Asian subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct01815424 (first received 21 March 2013). [CENTRAL: CN-01541153]
-
- Zhang J, Tsai TF, Lee MG, Zheng M, Wang G, Jin H, et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: a phase 3, randomized, double-blind, placebo-controlled study. Journal of Dermatological Science 2017;88(1):36-45. [CENTRAL: CN-01604532] - PubMed
Zhang 2020 {published data only}
-
- Zhang C, Yan K, Diao Q, Guo Q, Jin H, Yang S, et al. A multi-center, randomized, double-blind, placebo-controlled dose-ranging study evaluating efficacy and safety of SHR-1314 in subjects with moderate-to-severe plaque psoriasis. Arthritis & Rheumatology 2020;72 Suppl 10:1730-3.
Zhang 2022 {published data only}
-
- Zhang C, Yan K, Diao Q, Guo Q, Jin H, Yang S, et al. A multicenter, randomized, double-blinded, placebo-controlled, dose-ranging study evaluating the efficacy and safety of vunakizumab in patients with moderate-to-severe plaque psoriasis. Journal of the American Academy of Dermatology 2022;87(1):95-102. - PubMed
Zhu 2009 {published data only}
-
- Zhu Y, Hu C, Lu M, Liao S, Marini JC, Yohrling J, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. Journal of Clinical Pharmacology 2009;49(2):162-75. [PMID: ] - PubMed
Zhuang 2016 {published data only}
-
- Zhuang Y, Calderon C, Marciniak SJ Jr, Bouman-Thio E, Wasfi Y, Szapary P, et al. First-in-human study to assess guselkumab (anti-IL-23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate-to-severe psoriasis. European Journal of Clinical Pharmacology 2016;72(11):1303-10. [CENTRAL: CN-01307156] - PubMed
Zobel 1987 {published data only}
-
- Zobel AF. Cyclosporin is being tested for treatment of psoriasis. American Druggist 1987;195(3):102. [EMBASE: 1987106040]
References to studies awaiting assessment
ChiCTR2000034243 {published data only}
-
- ChiCTR2000034243. Phase III clinical trial to evaluate efficacy and safety of HS626 and remicade in patients with chronic moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000034243 (first received 29 June 2020).
Chow 2015 {published data only}
-
- Chow C, Simpson MJ, Luger TA, Chubb H, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (part 1 of 2): change during therapy in Psoriasis Area and Severity Index, Static Physician's Global Assessment and Lattice System Physician's Global Assessment. Journal of the European Academy of Dermatology and Venereology 2015;29(7):1406-14. [PMID: ] - PubMed
-
- Chow C, Simpson MJ, Zang Z, Goldfarb MT, Tejasvi T, Ellis CN. Longitudinal effects of active therapy in a clinical trial on Psoriasis Area and Severity Index, Static Physician's Global Assessment and Lattice System-Physician's Global Assessment for assessing severity of psoriasis. British Journal of Dermatology 2011;165(6):e30. [EMBASE: 70610815]
-
- Simpson MJ, Chow C, Morgenstern H, Luger TA, Ellis CN. Comparison of three methods for measuring psoriasis severity in clinical studies (part 2 of 2): use of quality of life to assess construct validity of the Lattice System Physician's Global Assessment, Psoriasis Area and Severity Index and Static Physician's Global Assessment. Journal of the European Academy of Dermatology and Venereology 2015;29(7):1415-20. [PMID: ] - PubMed
CTRI/2015/05/005830 {unpublished data only}
-
- CTRI/2015/05/005830. Role of oral methotrexate, cyclosporine and acitretin in treatment of palmoplantar psoriasis (redcoloured, painful, itchy, fissured lesions on hands and feet) and psoriasis vulgaris (red coloured, scaly, itchy, elevated lesions on skin over body). ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10246 (first received 29 May 2015).
CTRI/2016/10/007345 {unpublished data only}
-
- CTRI/2016/10/007345. A randomized, double-blind, placebo-controlled, comparative, prospective, multicentre trial to assess efficacy and safety of apremilast tablets in subjects with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=16164&EncHid=&mo... (first received 20 October 2016).
CTRI/2020/10/028555 {published data only}
-
- CTRI/2020/10/028555. Study the efficacy and side effects of subcutaneous v/s oral methotrexate in the management of moderate to severe psoriasis and palmoplantar psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/10/028555 (first received 22 October 2020).
DRKS00000716 {unpublished data only}
-
- DRKS00000716. Regulatory T-cell function in psoriasis vulgaris. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00... (first received 9 February 2011).
EUCTR2010‐020168‐39‐DE {published data only}
-
- EUCTR2010-020168-39-DE. A randomised, double blind, placebo controlled efficacy and safety trial of different doses/dose regimens of FP187 compared to placebo in moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2010-020168-39-DE (first received 9 June 2010).
EUCTR2015‐005279‐25‐DE {published data only}
-
- EUCTR2015-005279-25-DE. A research study to evaluate the efficacy of LEO 32731 oral tablet formulation in patients with moderate to severe psoriasis vulgaris. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-005279-25-DE (first received 10 February 2016).
EUCTR2021‐003700‐41‐ES {published data only}
-
- EUCTR. A phase 2b multicenter, randomized, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis - FRONTIER 1: efficacy and safety of JNJ-77242113 in moderate to severe plaque psoriasis. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu... 2021.
Goldust 2019 {published data only}
-
- Goldust M. Depression and anxiety in patients with moderate-to-severe plaque psoriasis while on methotrexate plus adalimumab vs. methotrexate monotherapy. British Journal of Dermatology 2019;181(Suppl 1):190.
Han 2007 {published data only}
-
- Han L, Fang X, Huang Q, Yang QP, Fu WW, Zheng ZZ, et al. Analysis of the effect of recombinant human tumor necrosis factor receptor in the treatment of moderate to severe plaque psoriasis on PASI score. Journal of Clinical Dermatology 2007;36(11):730-2. [CENTRAL: CN-00708092]
JPRN‐jRCT2061210069 {published data only}
-
- JPRN-jRCT2061210069. A study to assess adverse events and disease activity with cedirogant (ABBV-157) in adult participants with moderate to severe psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-jRCT2061210069 (first received 14 January 2022).
KEEPsAKE‐1 {published data only}
-
- Papp K, Soliman AM, Kaufmann C, Barcomb L, Wang Z, White D, et al. 33236 Impact of risankizumab on improving symptoms and health-related quality of life and reducing fatigue and pain among psoriatic arthritis patients with moderate-to-severe skin involvement: evidence from 2 phase III trials. Journal of the American Academy of Dermatology 2022;87(3 Suppl):AB174.
KEEPsAKE‐2 {published data only}
-
- NCT03671148. A study comparing risankizumab to placebo in participants with active psoriatic arthritis including those who have a history of inadequate response or intolerance to biologic therapy(ies) (KEEPsAKE2). https://clinicaltrials.gov/ct2/show/NCT03671148 (first received 17 October 2022).
Krishna 2016 {unpublished data only}
-
- Krishna C, Parmar N. Improvement in the quality of life of patients with severe plaque psoriasis treated with systemic methotrexate: a prospective, randomized, open-label, parallel group study in 60 patients. Journal of the Dermatology Nurses' Association (Conference: 24th World Congress of Dermatology, Italy) 2020;12(2):No pagination.
-
- Krishna CV, Rao AV. Improvement in the quality of life of patients with severe plaque psoriasis treated with systemic methotrexate in fixed doses of 10 mg or 25 mg orally once weekly: a prospective, randomized, double-blind, parallel-group study. British Journal of Dermatology 2016;175(S1):65-6.
-
- NCT02248792. Quality of life of patients with psoriasis treated with methotrexate: prospective, randomized, double-blind, parallel group study. clinicaltrials.gov/ct2/show/NCT02248792 (first received 22 September 2014).
Makavos 2020 {published data only}
-
- Makavos G, Ikonomidis I, Andreadou I, Varoudi M, Kapniari I, Loukeri E, et al. Effects of interleukin 17A Inhibition on myocardial deformation and vascular function in psoriasis. Canadian Journal of Cardiology 2020;36(1):100-11. - PubMed
Mrowietz 2005 {published data only}
-
- Mrowietz U, Spellman M. Dimethyl fumarate (BG00012) as an oral therapy for moderate to severe psoriasis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Journal of Investigative Dermatology 2005;125(3 Suppl):A69. [CENTRAL: CN-00792615]
-
- Mrowietz U, Spellman MC. Results of a phase III study of a novel oral formulation of dimethylfumarate in the treatment of moderate to severe plaque psoriasis: efficacy, safety, and quality of life effects. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):187. [CENTRAL: CN-00602493]
-
- Ortonne JP, Van de Kerkhof P, Mrowietz U. A novel oral agent improves quality of life (QOL) in patients with plaque psoriasis. In: 4th European Association of Dermatology and Venereology (EADV) Spring Symposium; 9-12 February 2006; Saariselka, Lapland, Finland. 2006:P-013. [CENTRAL: CN-00602214]
NCT01088165 {published data only}
-
- NCT01088165. The influence of adalimumab on cardiovascular and metabolic risk in psoriasis. clinicaltrials.gov/show/nct01088165 (first received 17 March 2010).
NCT01558310 {unpublished data only}
-
- NCT01558310. A study to evaluate the effectiveness of Stelara™ (ustekinumab) in the treatment of scalp psoriasis. clinicaltrials.gov/ct2/show/NCT01558310 (first received 20 March 2012).
NCT02655705 {unpublished data only}
-
- NCT02655705. Comparison study of psoriasis severity assessment tools. clinicaltrials.gov/ct2/show/NCT02655705 (first received 4 January 2016).
NCT02701205 {published data only}
-
- NCT02701205. Safety and efficacy study of etanercept (Qiangke®) to treat moderate to severe plaque psoriasis. clinicaltrials.gov/show/nct02701205 (first received 8 March 2016).
NCT02714322 {published data only}
-
- EUCTR2014-003420-46-BG. A study to evaluate the similarity in efficacy and safety of mylan adalimumab (MYL-1401A) compared with humira® in subjects with moderate-to-severe chronic skin inflammatory disease. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-003420-46-BG (first received 19 June 2015).
-
- NCT02714322. MYL-1401A efficacy and safety comparability study to humira®. clinicaltrials.gov/show/nct02714322 (first received 21 March 2016).
NCT02829424 {published data only}
-
- NCT02829424. Multicenter randomized double blind controlled-study to assess the potential of methotrexate versus placebo to improve and maintain response to anti TNF- alpha agents in adult patients with moderate to severe psoriasis. clinicaltrials.gov/show/nct02829424 (first received 12 July 2016).
References to ongoing studies
Alexis 2022 {published data only}
-
- Alexis A, Bhutani T, McMichael AJ, Choi O, Chan D, Rowland K, et al. 694 Study design of a phase 3b, multicenter, randomized, double-blind, placebo-controlled trial of guselkumab (GUS) in patients with skin of color who have moderate to severe plaque and/or scalp psoriasis (VISIBLE). Journal of Investigative Dermatology 2022;142(8 Suppl):S119.
-
- NCT05272150. Study of guselkumab in skin of color participants with moderate-to-severe plaque and/or scalp psoriasis. clinicaltrials.gov/show/NCT05272150 (first received 9 March 2022).
ChiCTR2000029262 {published data only}
-
- ChiCTR2000029262. Clinical study on the relationship between SLC35 gene variation and psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000029262 (first received 20 January 2020).
ChiCTR2000036186 {published data only}
-
- ChiCTR2000036186. A multi-center clinical study of systemic treatment strategies for psoriasis in Chinese population. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000036186 (first received 21 August 2020).
ChiCTR2000039699 {published data only}
-
- ChiCTR2000039699. A phase 4 clinical study to evaluate the efficacy and safety of induction and maintenance therapy of brodalumab (KHK4827) in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000039699 (first received 6 November 2020).
ChiCTR2100045970 {published data only}
-
- ChiCTR2100045970. A phase 3,multicenter,randomized,double-blind study evaluating the efficacy and safety of QX001S compared with ustekinumab in subjects with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100045970 (first received 30 April 2021).
CTRI/2019/07/020274 {published data only}
-
- CTRI/2019/07/020274. To compare the effect of three drugs- methotrexate, apremilast and their combination in patients suffering from psoriasis vulgaris. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/07/020274 (first received 17 July 2019).
CTRI/2020/02/023107 {published data only}
-
- CTRI/2020/02/023107. A comparative clinical study to evaluate efficacy and safety of test drug in patients with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/02/023107 (first received 4 February 2020).
CTRI/2022/01/039825 {published data only}
-
- CTRI/2022/01/039825. Comparison of drug methotrexate against drugs methotrexate with apremilast in psoriasis disorder. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/01/039825 (first received 31 January 2022).
Dong 2020 {published data only}
EUCTR2017‐001615‐36‐DE {published data only}
-
- EUCTR2017-001615-36-DE. A double-blind study in subjects with moderate to severe plaque psoriasis to evaluate efficacy, safety, tolerability of four different dose levels of ABY-035 compared to placebo. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-001615-36-DE (first received 7 September 2017).
EUCTR2017‐001695‐26‐IT {published data only}
-
- EUCTR2017-001695-26-IT. Effectiveness and safety of biological drugs in psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2017-001695-26-IT (first received 4 November 2020).
EUCTR2018‐001238‐16‐FR {published data only}
-
- EUCTR2018-001238-16-FR. A study to evaluate further therapeutic strategies with guselkumab in patients with moderate-to-severe plaque-type psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-001238-16-FR (first received 10 July 2019).
-
- Eyerich K, Weisenseel P, Pinter A, Schakel K, Asadullah K, Wegner S, et al. IL-23 blockade with guselkumab potentially modifies psoriasis pathogenesis: rationale and study protocol of a phase 3b, randomised, double-blind, multicentre study in participants with moderate-to-severe plaque-type psoriasis (GUIDE). BMJ Open 2021;11(9):no pagination. - PMC - PubMed
EUCTR2020‐005205‐42‐DE {published data only}
-
- EUCTR2020-005205-42-DE. A study to investigate interchangeability of ABP 654 for the treatment of subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-005205-42-DE (first received 16 March 2021).
EUCTR2021‐003209‐22‐DE {published data only}
-
- EUCTR2021-003209-22-DE. Study to assess the efficacy and safety of orismilast in psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-003209-22-DE (first received 8 October 2021).
Holsken 2021 {published data only}
-
- DRKS00022104. Influence of psychological factors on the response to therapy with secukinumab in psoriasis patients on the subjective and objective level. www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00022104 (first received 9 June 2020).
IRCT20100102002954N26 {published data only}
-
- IRCT20100102002954N26. Comparison of the effect of adalimumab and methotrexate in the treatment of psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20100102002954N26 (first received 28 February 2022).
IRCT20120524009844N8 {published data only}
-
- IRCT20120524009844N8. Therapeutic effects of adalimumab in patients with resistant psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20120524009844N8 (first received 20 October 2020).
NCT02258282 {unpublished data only}
-
- NCT02258282. Safety and efficacy of etanercept in patients with psoriasis. clinicaltrials.gov/ct2/show/NCT02258282 (first received 7 October 2014).
NCT03478280 {published data only}
-
- NCT03478280. Effect of brodalumab compared to placebo on vascular inflammation in moderate-to-severe psoriasis. clinicaltrials.gov/show/nct03478280 (first received 27 March 2018).
NCT03897075 {published data only}
-
- NCT03897075. Efficacy and safety study of tildrakizumab in the treatment of nail psoriasis. clinicaltrials.gov/show/nct03897075 (first received 1 April 2019).
NCT03897088 {published data only}
-
- NCT03897088. Efficacy and safety of tildrakizumab in the treatment of scalp psoriasis. clinicaltrials.gov/show/nct03897088 (first received 1 April 2019).
NCT03927352 {published data only}
-
- NCT03927352. Efficacy and safety of SCT630 and adalimumab (HUMIRA®) in adults with plaque psoriasis. clinicaltrials.gov/show/nct03927352 (first received 25 April 2019).
NCT04102241 {published data only}
-
- NCT04102241. Efficacy and safety study of hemay005 in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04102241 (first received 25 September 2019).
NCT04237116 {published data only}
-
- EUCTR2019-003168-37-DE. Study in patients with plaque psoriasis with coexisting non-alcoholic fatty liver disease. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-003168-37-DE (first received 24 October 2019).
-
- NCT04237116. A study of secukinumab treatment in patients with plaque psoriasis and coexisting non-alcoholic fatty liver disease (NAFLD) (pINPOINt). clinicaltrials.gov/show/NCT04237116 (first received 23 January 2020).
NCT04306315 {published data only}
-
- EUCTR2017-004998-13-FR. Adjusted brodalumab dose compared with standard brodalumab dose in subjects with moderate-to-severe plaque psoriasis and ≥120 kg body weight. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-004998-13-FR (first received 10 April 2020).
-
- NCT04306315. Adjusted brodalumab dose compared with standard brodalumab dose in subjects with moderate-to-severe plaque psoriasis and ≥120 kg body weight. clinicaltrials.gov/show/NCT04306315 (first received 12 March 2020).
NCT04394936 {published data only}
-
- EUCTR2019-002383-27-NL. An explorative psoriasis biomarker study. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-002383-27-NL (first received 13 June 2019).
-
- NCT04394936. An explorative psoriasis biomarker study. clinicaltrials.gov/show/NCT04394936 (first received 20 May 2020).
NCT04453137 {published data only}
-
- EUCTR2019-002911-25-PL. Multicenter, double-blind, randomized, parallel-group, study evaluating pharmacokinetic, efficacy, safety, and immunogenicity between patients with moderate to severe chronic plaque psoriasis receiving humira® and patients undergoing repeated switches between humira® and AVT02 followed by a safety extension phase of AVT02. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-002911-25-PL (first received 28 June 2020).
-
- NCT04453137. Pharmacokinetic, efficacy, safety, and immunogenicity of AVT02 with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT04453137 (first received 1 July 2020).
NCT04533737 {published data only}
-
- NCT04533737. Efficacy and safety of brodalumab compared with guselkumab in the treatment of plaque psoriasis after inadequate response to ustekinumab (COBRA). clinicaltrials.gov/show/NCT04533737 (first received 1 September 2020).
NCT04595409 {published data only}
-
- EUCTR2019-004364-21-PL. A double-blind study to compare the efficacy, safety, and immunogenicity of the proposed biosimilar ustekinumab FYB202 to stelara in patients with moderate-to-severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-004364-21-PL (first received 5 March 2020).
-
- NCT04595409. A double-blind study to compare the efficacy, safety, and immunogenicity of the proposed biosimilar ustekinumab FYB202 to stelara® in patients with moderate-to-severe plaque psoriasis (VESPUCCI). clinicaltrials.gov/show/NCT04595409 (first received 20 October 2020).
NCT04607980 {published data only}
-
- EUCTR2020-003184-25-EE. A study to investigate ABP 654 for the treatment of subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-003184-25-EE (first received 6 November 2020).
-
- NCT04607980. A study to investigate ABP 654 for the treatment of participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04607980 (first received 29 October 2020).
NCT04673786 {published data only}
-
- EUCTR2020-001045-39-EE. Study to compare the efficacy and safety of CT-P43 to Stelara in patients with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-001045-39-EE (first received 1 October 2020).
-
- NCT04673786. A phase 3 study to compare the efficacy and safety of CT-P43 to Stelara in patients with plaque psoriasis. clinicaltrials.gov/show/NCT04673786 (first received 17 December 2020).
NCT04713592 {published data only}
-
- NCT04713592. Study of subcutaneous (injected under the skin) risankizumab to assess change in disease symptoms in adult participants with moderate to severe plaque psoriasis with palmoplantar involvement. clinicaltrials.gov/show/NCT04713592 (first received 19 January 2021).
NCT04728360 {published data only}
-
- EUCTR2020-004504-33-BG. Study to compare efficacy and safety of BAT2206 with Stelara® in patients with moderate to severe plaque psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-004504-33-BG (first received 9 February 2021).
-
- NCT04728360. Comparative study of BAT2206 with Stelara® in patients with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04728360 (first received 28 January 2021).
NCT04785326 {published data only}
-
- EUCTR2020-005108-21-HU. Clinical study comparing DMB-3115 and Stelara® in patients with moderate to severe chronic plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-005108-21-HU (first received 1 March 2021).
-
- NCT04785326. Efficacy, safety, and immunogenicity of subcutaneous DMB-3115 versus stelara® in patients with moderate to severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT04785326 (first received 5 March 2021).
NCT04839328 {published data only}
-
- NCT04839328. A phase Ⅲ efficacy and safety study of hemay005 in subjects with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04839328 (first received 9 April 2021).
NCT04908475 {published data only}
-
- EUCTR2020-005512-21-DE. Study of subcutaneous risankizumab injection compared to oral apremilast tablets to assess adverse events and change in disease activity in adult participants with moderate plaque psoriasis who are candidates for systemic therapy. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-005512-21-DE (first received 7 May 2021).
-
- NCT04908475. Study of subcutaneous risankizumab injection compared to oral apremilast tablets to assess change in disease activity and adverse events in adult participants with moderate plaque psoriasis who are candidates for systemic therapy. clinicaltrials.gov/show/NCT04908475 (first received 1 June 2021).
NCT04914429 {published data only}
-
- NCT04914429. A study of guselkumab (TREMFYA) in Chinese participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04914429 (first received 4 June 2021).
NCT04930042 {published data only}
-
- EUCTR2020-004493-22-PL. A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar ustekinumab (AVT04) and Stelara® in patients with moderate to severe chronic plaque-type psoriasis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-004493-22-PL (first received 29 January 2021).
-
- NCT04930042. Efficacy, safety, and immunogenicity of AVT04 with moderate-to-severe chronic plaque psoriasis. clinicaltrials.gov/show/NCT04930042 (first received 18 June 2021).
NCT04967508 {published data only}
-
- EUCTR2020-006115-19-CZ. SB17 versus Stelara® in subjects with moderate to severe plaque psoriasis. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-006115-19-CZ (first received 31 March 2021).
-
- NCT04967508. A study to compare SB17 (proposed ustekinumab biosimilar) to Stelara® in subject with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT04967508 (first received 19 July 2021).
NCT05004727 {published data only}
-
- NCT05004727. Multi-center PAMPA study. clinicaltrials.gov/show/NCT05004727 (first received 13 August 2021).
NCT05020249 {published data only}
-
- NCT05020249. A study to evaluate the efficacy and safety of bimekizumab in adult Korean study participants with moderate to severe plaque psoriasis. clinicaltrials.gov/show/NCT05020249 (first received 25 August 2021).
NCT05108766 {published data only}
-
- NCT05108766. A phase Ⅲ study to evaluate tildrakizumab in the treatment of Chinese subjects with moderate to severe plaquetype psoriasis. clinicaltrials.gov/show/NCT05108766 (first received 5 November 2021).
NCT05335356 {published data only}
-
- NCT05335356. Comparing efficacy and safety of Bmab 1200 and Stelara in patients with moderate to severe chronic plaque psoriasis (STELLAR-2). clinicaltrials.gov/show/NCT05335356 (first received 19 April 2022).
NCT05344248 {published data only}
-
- NCT05344248. Safety, tolerance, efficacy and pharmacokinetics of JS005 multiple dosing. clinicaltrials.gov/show/NCT05344248 (first received 25 April 2022).
NCT05536726 {published data only}
-
- NCT05536726. A phase 3 study of recombinant anti-IL-17A humanized monoclonal antibody in Chinese participants with moderate-to-severe plaque psoriasis. clinicaltrials.gov/show/NCT05536726 (first received 13 September 2022).
Additional references
Afach 2021
Afach 2022
Alinaghi 2019
-
- Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. Journal of the American Academy of Dermatology 2019;80(1):251-65.e19. [PMID: ] - PubMed
Aljefri 2022
-
- Aljefri Yara E, Ghaddaf Abdullah A, Alkhunani Tala A, Alkhamisi Taif A, Alahmadi Rana A, Alamri Awadh M, Alraddadi Ali A. Efficacy and safety of apremilast monotherapy in moderate-to-severe plaque psoriasis: A systematic review and meta-analysis. Dermatologic Therapy 2022;35(7):e15544. - PubMed
Armstrong 2020b
-
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020;323(19):1945-60. [PMID: ] - PubMed
Armstrong 2021
Armstrong 2022
Armstrong 2022a
Atwan 2015
Balak 2016
-
- Balak DM, Fallah Arani S, Hajdarbegovic E, Hagemans CA, Bramer WM, Thio HB, et al. Efficacy, effectiveness and safety of fumaric acid esters in the treatment of psoriasis: a systematic review of randomized and observational studies. British Journal of Dermatology 2016;175(2):250-62. [PMID: ] - PubMed
Bansback 2009
-
- Bansback N, Sizto S, Sun H, Feldman S, Willian MK, Anis A. Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta-analysis. Dermatology 2009;219(3):209-18. [MEDLINE: ] - PubMed
Blauvelt 2021b
Blauvelt 2022
Brimhall 2008
-
- Brimhall AK, King LN, Licciardone JC, Jacobe H, Menter A. Safety and efficacy of alefacept, efalizumab, etanercept and infliximab in treating moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. British Journal of Dermatology 2008;159(2):274-85. [MEDLINE: ] - PubMed
Callis Duffin 2018
Campanati 2017
-
- Campanati A, Benfaremo D, Luchetti MM, Ganzetti G, Gabrielli A, Offidani A. Certolizumab pegol for the treatment of psoriasis. Expert Opinion on Biological Therapy 2017;17(3):387-94. [PMID: ] - PubMed
Capon 2017
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. [PMID: ] - PubMed
Chaimani 2013
-
- Chaimani A, Vasiliadis HS, Pandis N, Schmid CH, Welton NJ, Salanti G. Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. International Journal of Epidemiology 2013;42(4):1120-31. [PMID: ] - PubMed
Chaimani 2017a
-
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;16(S0895):30775-2. [PMID: ] - PubMed
Chaimani 2017b
-
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. - PubMed
Chaimani 2017c
Chiu 2014
-
- Chiu HY, Wang TS, Chan CC, Cheng YP, Lin SJ, Tsai TF. Human leucocyte antigen-Cw6 as a predictor for clinical response to ustekinumab, an interleukin-12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. British Journal of Dermatology 2014;171(5):1181-8. [PMID: ] - PubMed
Christophers 1992
-
- Christophers E, Mrowietz U, Henneicke HH, Farber L, Welzel D. Cyclosporine in psoriasis: a multicenter dose-finding study in severe plaque psoriasis. The German Multicenter Study. Journal of the American Academy of Dermatology 1992;26(1):86-90. [PMID: ] - PubMed
CINeMA 2017 [Computer program]
-
- CINeMA: Confidence in Network Meta-Analysis. Bern: Institute of Social and Preventive Medicine, University of Bern, 2017.
Cipriani 2013
-
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [PMID: ] - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, N.J: L. Erlbaum Associates, 1988.
Covidence 2021 [Computer program]
-
- Covidence. Version accessed prior to 6 October 2021. Melbourne, Australia: Veritas Health Innovation, 2014. Available at covidence.org.
Dechartres 2013
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29(7-8):932-44. [PMID: ] - PubMed
Dong 2017
-
- Dong J, Goldenberg G. New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors. Cutis 2017;99(2):123-7. [PMID: ] - PubMed
Elliott 2017
-
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. Journal of Clinical Epidemiology 2017;91:23-30. - PubMed
Evrenoglou 2022
Fahrbach 2021
-
- Fahrbach K, Sarri G, Phillippo DM, Neupane B, Martel SE, Kiri S, et al. Short-term efficacy of biologic therapies in moderate-to-severe plaque psoriasis: a systematic literature review and an enhanced multinomial network meta-analysis. Dermatology and Therapy 2021;11(6):1965-98. [PMID: ] - PMC - PubMed
Fu 2022
Gisondi 2004
-
- Gisondi P, Gubinelli E, Cocuroccia B, Girolomoni G. Targeting tumor necrosis factor-alpha in the therapy of psoriasis. Current Drug Targets. Inflammation and Allergy 2004;3(2):175-83. [PMID: ] - PubMed
Gospodarevskaya 2009
-
- Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A. Ustekinumab for the treatment of moderate to severe psoriasis. Health Technology Assessment 2009;13(Suppl 3):61-6. [MEDLINE: ] - PubMed
Gottlieb 2022
-
- Gottlieb AB, Saure D, Wilhelm S, Dossenbach M, Schuster C, Smith SD, et al. Indirect comparisons of ixekizumab versus three interleukin-23 p19 inhibitors in patients with moderate-to-severe plaque psoriasis - efficacy findings up to week 12. Journal of Dermatological Treatment 2022;33(1):54-61. - PubMed
Griffiths 2007
-
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370(9583):263-71. [MEDLINE: ] - PubMed
Grodner 2021
-
- Grodner C, Sbidian E, Weill A, Mezzarobba M. Epidemiologic study in a real-world analysis of patients with treatment for psoriasis in the French national health insurance database. Journal of the European Academy of Dermatology and Venereology 2021;35(2):411-6. - PubMed
Guelimi 2021
Gómez‐García 2017
-
- Gómez-García F, Epstein D, Isla-Tejera B, Lorente A, Vélez García-Nieto A, Ruano J. Short-term efficacy and safety of new biological agents targeting the interleukin-23-T helper 17 pathway for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. British Journal of Dermatology 2017;176(3):594-603. [PMID: ] - PubMed
He 2021
Helliwell 2005
Higgins 2012
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Ho 1996
-
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. Clinical Immunology and Immunopathology 1996;80(3 Pt 2):S40-5. [MEDLINE: ] - PubMed
Ho 1999
-
- Ho VC, Griffiths CE, Albrecht G, Vanaclocha F, Leon-Dorantes G, Atakan N, et al. Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study. The PISCES Study Group. British Journal of Dermatology 1999;141(2):283-91. [MEDLINE: ] - PubMed
Jackson 2014
Kang 2022
Kimball 2005
-
- Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. American Journal of Clinical Dermatology 2005;6(6):383-92. [MEDLINE: ] - PubMed
Langan 2012
Le Cleach 2008
-
- Le Cleach L, Chassany O, Levy A, Wolkenstein P, Chosidow O. Poor reporting of quality of life outcomes in dermatology randomized controlled clinical trials. Dermatology 2008;216(1):46-55. [MEDLINE: ] - PubMed
Lebwohl 2010
-
- Lebwohl M, Papp K, Han C, Schenkel B, Yeilding N, Wang Y, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. British Journal of Dermatology 2010;162(1):137-46. [MEDLINE: ] - PubMed
Leonardi 2022
Liang 2019
-
- Liang F, Wu Z, Mo M, Zhou C, Shen J, Wang Z, et al. Comparison of treatment effect from randomised controlled phase II trials and subsequent phase III trials using identical regimens in the same treatment setting. European Journal of Cancer 2019;121:19-28. - PubMed
Lin 2012
-
- Lin VW, Ringold S, Devine EB. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis: a Bayesian network meta-analysis. Archives of Dermatology 2012;148(12):1403-10. [MEDLINE: ] - PubMed
Living Evidence Network 2019
-
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Available from community.cochrane.org/review-production/production-resources/living-sys... (accessed prior to 4 April 2022).
Loveman 2009
-
- Loveman E, Turner D, Hartwell D, Cooper K, Clegg A. Infliximab for the treatment of adults with psoriasis. Health Technology Assessment 2009;13(Suppl 1):55-60. [MEDLINE: ] - PubMed
Marshall 2018
Mason 2013
Mavridis 2014
-
- Mavridis D, Chaimani A, Efthimiou O, Leucht S, Salanti G. Addressing missing outcome data in meta-analysis. Evidence-Based Mental Health 2014;17(3):85-9. [PMID: ] - PubMed
Maza 2011
-
- Maza A, Montaudie H, Sbidian E, Gallini A, Aractingi S, Aubin F, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. Journal of the European Academy of Dermatology and Venereology 2011;25 Suppl 2:19-27. [PMID: ] - PubMed
Montaudie 2011
-
- Montaudie H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S, et al. Methotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. Journal of the European Academy of Dermatology and Venereology 2011;25 Suppl 2:12-8. [PMID: ] - PubMed
Mossner 2009
-
- Mossner R, Reich K. Management of severe psoriasis with TNF antagonists. Adalimumab, etanercept and infliximab. Current Problems in Dermatology 2009;38:107-36. [PMID: ] - PubMed
Mrowietz 1995
-
- Mrowietz U, Farber L, Henneicke-von Zepelin HH, Bachmann H, Welzel D, Christophers E. Long-term maintenance therapy with cyclosporine and posttreatment survey in severe psoriasis: results of a multicenter study. German Multicenter Study. Journal of the American Academy of Dermatology 1995;33(3):470-5. [PMID: ] - PubMed
Mrowietz 2021
-
- Mrowietz U, Warren RB, Leonardi CL, Saure D, Petto H, Hartz S, et al. Network meta-analysis of biologic treatments for psoriasis using absolute Psoriasis Area and Severity Index values ≤ 1, 2, 3 or 5 derived from a statistical conversion method. Journal of the European Academy of Dermatology and Venereology 2021;35(5):1161-75. [PMID: ] - PMC - PubMed
Naldi 2010
-
- Naldi L. Scoring and monitoring the severity of psoriasis. What is the preferred method? What is the ideal method? Is PASI passe? Facts and controversies. Clinics in Dermatology 2010;28(1):67-72. [MEDLINE: ] - PubMed
Nast 2015a
-
- Nast A, Jacobs A, Rosumeck S, Werner RN. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. Journal of Investigative Dermatology 2015;135(11):2641-8. [PMID: ] - PubMed
Nast 2015b
-
- Nast A, Jacobs A, Rosumeck S, Werner RN. Methods Report: European S3-Guidelines on the systemic treatment of psoriasis vulgaris - update 2015--EDF in cooperation with EADV and IPC. Journal of the European Academy of Dermatology and Venereology 2015;29(12):e1-22. [DOI: 10.1111/jdv.13353] - DOI - PubMed
Nelson 2008
-
- Nelson AA, Pearce DJ, Fleischer AB Jr, Balkrishnan R, Feldman SR. Cost-effectiveness of biologic treatments for psoriasis based on subjective and objective efficacy measures assessed over a 12-week treatment period. Journal of the American Academy of Dermatology 2008;58(1):125-35. [MEDLINE: ] - PubMed
Nijsten 2007
-
- Nijsten T, Looman CW, Stern RS. Clinical severity of psoriasis in last 20 years of PUVA study. Archives of Dermatology 2007;143(9):1113-21. [MEDLINE: ] - PubMed
Noel‐Storr 2021
Ormerod 2004
-
- Ormerod AD, Mrowietz U. Fumaric acid esters, their place in the treatment of psoriasis. British Journal of Dermatology 2004;150(4):630-2. [MEDLINE: ] - PubMed
Pan 2021
Parisi 2020
Rapp 1999
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology 1999;41(3 Pt 1):401-7. [MEDLINE: ] - PubMed
Reich 2008
-
- Reich K, Sinclair R, Roberts G, Griffiths CE, Tabberer M, Barker J. Comparative effects of biological therapies on the severity of skin symptoms and health-related quality of life in patients with plaque-type psoriasis: a meta-analysis. Current Medical Research and Opinion 2008;24(5):1237-54. [MEDLINE: ] - PubMed
Reich 2012b
-
- Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. British Journal of Dermatology 2012;166(1):179-88. [MEDLINE: ] - PubMed
Revman 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4.1. Nordic Cochrane Centre, The Cochrane Collaboration, 2020. Copenhagen.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ (Clinical research ed.) 2011;342:d549. [PMID: ] - PubMed
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [MEDLINE: ] - PubMed
Salanti 2014
Savage 2015
Sawyer 2019
-
- Sawyer LM, Cornic L, Levin LÅ, Gibbons C, Møller AH, Jemec GB. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. Journal of the European Academy of Dermatology and Venereology 2019;33(2):355-66. [PMID: ] - PMC - PubMed
Sbidian 2011
-
- Sbidian E, Maza A, Montaudie H, Gallini A, Aractingi S, Aubin F, et al. Efficacy and safety of oral retinoids in different psoriasis subtypes: a systematic literature review. Journal of the European Academy of Dermatology and Venereology 2011;25 Suppl 2:28-33. [PMID: ] - PubMed
Schmitt 2005
-
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 2005;210(3):194-9. [PMID: ] - PubMed
Schmitt 2008
-
- Schmitt J, Zhang Z, Wozel G, Meurer M, Kirch W. Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. British Journal of Dermatology 2008;159(3):513-26. [PMID: ] - PubMed
Shear 2021
-
- Shear NH, Betts KA, Soliman AM, Joshi A, Wang Y, Zhao J, et al. Comparative safety and benefit-risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: a network meta-analysis of clinical trial data. Journal of the American Academy of Dermatology 2021;85(3):572-81. [PMID: ] - PubMed
Signorovitch 2010
-
- Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics 2010;28(10):935-45. [MEDLINE: ] - PubMed
Signorovitch 2015
-
- Signorovitch JE, Betts KA, Yan YS, LeReun C, Sundaram M, Wu EQ, et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. British Journal of Dermatology 2015;172(2):504-12. [PMID: ] - PubMed
Song 2021
-
- Song GG, Lee YH. Relative efficacy and safety of tofacitinib for treating psoriasis: a Bayesian network meta-analysis of randomized controlled trials. International Journal of Clinical Pharmacology and Therapeutics 2021;59(4):308-14. [PMID: ] - PubMed
Spuls 1997
-
- Spuls PI, Witkamp L, Bossuyt PM, Bos JD. A systematic review of five systemic treatments for severe psoriasis. British Journal of Dermatology 1997;137(6):943-9. [MEDLINE: ] - PubMed
Spuls 2010
-
- Spuls PI, Lecluse LL, Poulsen ML, Bos JD, Stern RS, Nijsten T. How good are clinical severity and outcome measures for psoriasis?: quantitative evaluation in a systematic review. Journal of Investigative Dermatology 2010;130(4):933-43. [MEDLINE: ] - PubMed
Strober 2006
-
- Strober BE, Siu K, Menon K. Conventional systemic agents for psoriasis. A systematic review. Journal of Rheumatology 2006;33(7):1442-6. [MEDLINE: ] - PubMed
Tan 2011
-
- Tan JY, Li S, Yang K, Ma B, Chen W, Zha C, et al. Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: a meta-analysis. Journal of Dermatological Treatment 2011;22(6):323-36. [MEDLINE: ] - PubMed
Torres 2015
-
- Torres T, Filipe P. Small molecules in the treatment of psoriasis. Drug Development Research 2015;76(5):215-27. [PMID: ] - PubMed
Tubach 2009
-
- Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Breban M, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis and Rheumatism 2009;60(7):1884-94. [MEDLINE: ] - PMC - PubMed
Turner 2009
-
- Turner D, Picot J, Cooper K, Loveman E. Adalimumab for the treatment of psoriasis. Health Technology Assessment 2009;13(Suppl 2):49-54. [MEDLINE: ] - PubMed
Veroniki 2013
Veroniki 2018
-
- Veroniki AA, Straus SE, Rücker G, Tricco AC. Is providing uncertainty intervals in treatment ranking helpful in a network meta-analysis? Journal of Clinical Epidemiology 2018;100:122-9. - PubMed
White 2012
Woolacott 2006
-
- Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Vergel YB, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technology Assessment 2006;10(46):1-233, i-iv. [MEDLINE: ] - PubMed
Xie 2021
-
- Xie Yan, Liu Yang, Liu Yi. Are biologics combined with methotrexate better than biologics monotherapy in psoriasis and psoriatic arthritis: a meta-analysis of randomized controlled trials. Dermatologic Therapy 2021;34(3):e14926. - PubMed
Xu 2021
-
- Xu S, Gao X, Deng J, Yang J, Pan F. Comparative efficacy and safety of biologics in moderate to severe plaque psoriasis: a multiple-treatments meta-analysis. Journal der Deutschen Dermatologischen Gesellschaft (Journal of the German Society of Dermatology : JDDG) 2021;19(1):47-56. [PMID: ] - PubMed
Yan 2021
Yan 2021a
Yasmeen 2022
-
- Yasmeen N, Sawyer LM, Malottki K, Levin LÅ, Didriksen AE, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. Journal of Dermatological Treatment 2022;33(1):204-18. - PubMed
Yu 2022
Zachariae 2003
-
- Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. American Journal of Clinical Dermatology 2003;4(7):441-7. [MEDLINE: ] - PubMed
References to other published versions of this review
Sbidian 2015
-
- Sbidian E, Le Cleach L, Trinquart L, Do G, Hughes C, Naldi L, et al. Systemic pharmacological treatments for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD011535. [DOI: 10.1002/14651858.CD011535] - DOI
Sbidian 2017
Sbidian 2020
Sbidian 2021
Sbidian 2022
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

