EMS Administration of Systemic Corticosteroids to Pediatric Asthma Patients: An Analysis by Severity and Transport Interval
- PMID: 37428954
- PMCID: PMC10592383
- DOI: 10.1080/10903127.2023.2234996
EMS Administration of Systemic Corticosteroids to Pediatric Asthma Patients: An Analysis by Severity and Transport Interval
Abstract
Introduction: Pediatric asthma exacerbations are a common cause of emergency medical services (EMS) encounters. Bronchodilators and systemic corticosteroids are mainstays of asthma exacerbation therapy, yet data on the efficacy of EMS administration of systemic corticosteroids are mixed. This study's objective was to assess the association between EMS administration of systemic corticosteroids to pediatric asthma patients on hospital admission rates based on asthma exacerbation severity and EMS transport intervals.
Methods: This is a sub-analysis of the Early Administration of Steroids in the Ambulance Setting: An Observational Design Trial (EASI AS ODT). EASI AS ODT is a non-randomized, stepped wedge, observational study examining outcomes one year before and one year after seven EMS agencies incorporated an oral systemic corticosteroid option into their protocols for the treatment of pediatric asthma exacerbations. We included EMS encounters for patients ages 2-18 years confirmed by manual chart review to have asthma exacerbations. We compared hospital admission rates across asthma exacerbation severities and EMS transport intervals using univariate analyses. We geocoded patients and created maps to visualize the general trends of patient characteristics.
Results: A total of 841 pediatric asthma patients met inclusion criteria. While most patients were administered inhaled bronchodilators by EMS (82.3%), only 21% received systemic corticosteroids, and only 19% received both inhaled bronchodilators and systemic corticosteroids. Overall, there was no significant difference in hospitalization rates between patients who did and did not receive systemic corticosteroids from EMS (33% vs. 32%, p = 0.78). However, although not statistically significant, for patients who received systemic corticosteroids from EMS, there was an 11% decrease in hospitalizations for mild exacerbation patients and a 16% decrease in hospitalizations for patients with EMS transport intervals greater than 40 min.
Conclusion: In this study, systemic corticosteroids were not associated with a decrease in hospitalizations of pediatric patients with asthma overall. However, while limited by small sample size and lack of statistical significance, our results suggest there may be a benefit in certain subgroups, particularly patients with mild exacerbations and those with transport intervals longer than 40 min. Given the heterogeneity of EMS agencies, EMS agencies should consider local operational and pediatric patient characteristics when developing standard operating protocols for pediatric asthma.
Conflict of interest statement
Figures
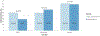
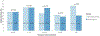
Similar articles
-
Evaluation of the implementation of evidence-based pediatric asthma exacerbation treatments in a regional consortium of emergency medical Services Agencies.J Asthma. 2024 May;61(5):405-416. doi: 10.1080/02770903.2023.2280917. Epub 2023 Nov 11. J Asthma. 2024. PMID: 37930329
-
Prehospital Pediatric Asthma Care during COVID-19: Changes to EMS Treatment Protocols and Downstream Clinical Effects.Prehosp Emerg Care. 2023;27(7):893-899. doi: 10.1080/10903127.2022.2137864. Epub 2022 Nov 8. Prehosp Emerg Care. 2023. PMID: 36260781 Free PMC article.
-
Early administration of steroids in the ambulance setting: Protocol for a type I hybrid effectiveness-implementation trial with a stepped wedge design.Contemp Clin Trials. 2020 Oct;97:106141. doi: 10.1016/j.cct.2020.106141. Epub 2020 Sep 12. Contemp Clin Trials. 2020. PMID: 32931918 Free PMC article.
-
Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children.Cochrane Database Syst Rev. 2013 Apr 30;2013(4):CD007313. doi: 10.1002/14651858.CD007313.pub3. Cochrane Database Syst Rev. 2013. PMID: 23633340 Free PMC article. Review.
-
Combination of inhaled long-acting beta2-agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD005533. doi: 10.1002/14651858.CD005533. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2010 Apr 14;(4):CD005533. doi: 10.1002/14651858.CD005533.pub2. PMID: 16235409 Updated. Review.
Cited by
-
Evaluation of the implementation of evidence-based pediatric asthma exacerbation treatments in a regional consortium of emergency medical Services Agencies.J Asthma. 2024 May;61(5):405-416. doi: 10.1080/02770903.2023.2280917. Epub 2023 Nov 11. J Asthma. 2024. PMID: 37930329
-
Early Administration of Steroids in the Ambulance Setting: An Observational Design Trial (EASI-AS-ODT).Acad Emerg Med. 2024 Jan;31(1):49-60. doi: 10.1111/acem.14813. Epub 2023 Oct 19. Acad Emerg Med. 2024. PMID: 37786991 Free PMC article.
-
Development of a Computable Phenotype for Prehospital Pediatric Asthma Encounters.Prehosp Emerg Care. 2025;29(1):10-21. doi: 10.1080/10903127.2024.2352583. Epub 2024 May 21. Prehosp Emerg Care. 2025. PMID: 38713633
References
-
- McCaig LF, Nawar EN. National Hospital Ambulatory Medical Care Survey: 2004 emergency department summary. Advance data from vital and health statistics; no 372. Hyattsville, MD: National Center for Health Statistics. 2006. - PubMed
-
- Lerner EB, Dayan PS, Brown K, Fuchs S, Leonard J, Borgialli D, Babcock L, Hoyle JD Jr, Kwok M, Lillis K, et al.; Pediatric Emergency Care Applied Research Network (PECARN). Characteristics of the pediatric patients treated by the Pediatric Emergency Care Applied Research Network’s affiliated EMS agencies. Prehosp Emerg Care. 2014;18(1):52–9. - PubMed
-
- Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, Dixon AE, Elward KS, Hartert T, Krishnan JA, et al.; Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC). 2020 focused updates to the Asthma Management Guidelines: A report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. Journal of Allergy and Clinical Immunology. 2020;146(6):1217–1270. doi:10.1016/j.jaci.2020.10.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
