CD24 is a novel target of chimeric antigen receptor T cells for the treatment of triple negative breast cancer
- PMID: 37418008
- PMCID: PMC10991104
- DOI: 10.1007/s00262-023-03491-7
CD24 is a novel target of chimeric antigen receptor T cells for the treatment of triple negative breast cancer
Abstract
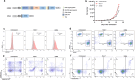
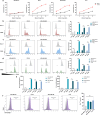
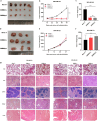
Triple negative breast cancer (TNBC) is a subtype of breast cancer with the highest degree of malignancy and the worst prognosis. The application of immunotherapy for TNBC is limited. This study was to verify the potential application of chimeric antigen receptor-T cells (CAR-T cells) targeting CD24 named as 24BBz in treatment of TNBC. 24BBz was constructed by lentivirus infection and then was co-culture with breast cancer cell lines to evaluate the activation, proliferation and cytotoxicity of engineered T cells. The anti-tumor activity of 24BBz was verified in the subcutaneous xenograft model of nude mice. We found that CD24 gene was significantly up-regulated in breast cancer (BRCA), especially in TNBC. 24BBz showed antigen-specific activation and dose-dependent cytotoxicity against CD24-positive BRCA tumor cells in vitro. Furthermore, 24BBz showed significant anti-tumor effect in CD24-positive TNBC xenografts and T cells infiltration in tumor tissues, while some T cells exhibited exhaustion. No pathological damage of major organs was found during the treatment. This study proved that CD24-specific CAR-T cells have potent anti-tumor activity and potential application value in treatment of TNBC.
Keywords: CAR-T cell therapy; CD24 antigen; Solid tumor; TNBC.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor.Cell Prolif. 2020 Aug;53(8):e12858. doi: 10.1111/cpr.12858. Epub 2020 Jun 27. Cell Prolif. 2020. PMID: 32592435 Free PMC article.
-
A novel AXL chimeric antigen receptor endows T cells with anti-tumor effects against triple negative breast cancers.Cell Immunol. 2018 Sep;331:49-58. doi: 10.1016/j.cellimm.2018.05.004. Epub 2018 May 14. Cell Immunol. 2018. PMID: 29935762
-
CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth.Front Immunol. 2019 May 24;10:1149. doi: 10.3389/fimmu.2019.01149. eCollection 2019. Front Immunol. 2019. PMID: 31178870 Free PMC article.
-
Emerging CAR-T Cell Therapy for the Treatment of Triple-Negative Breast Cancer.Mol Cancer Ther. 2020 Dec;19(12):2409-2421. doi: 10.1158/1535-7163.MCT-20-0385. Epub 2020 Oct 21. Mol Cancer Ther. 2020. PMID: 33087511 Review.
-
Novel chimeric antigen receptor T cell-based immunotherapy: a perspective for triple-negative breast cancer.Front Cell Dev Biol. 2023 Jun 29;11:1158539. doi: 10.3389/fcell.2023.1158539. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37457288 Free PMC article. Review.
Cited by
-
Engineered Adoptive T-Cell Therapies for Breast Cancer: Current Progress, Challenges, and Potential.Cancers (Basel). 2023 Dec 26;16(1):124. doi: 10.3390/cancers16010124. Cancers (Basel). 2023. PMID: 38201551 Free PMC article. Review.
-
Checkpoint CD24 function on tumor and immunotherapy.Front Immunol. 2024 Feb 29;15:1367959. doi: 10.3389/fimmu.2024.1367959. eCollection 2024. Front Immunol. 2024. PMID: 38487533 Free PMC article. Review.
-
From mechanism to therapy: the journey of CD24 in cancer.Front Immunol. 2024 May 31;15:1401528. doi: 10.3389/fimmu.2024.1401528. eCollection 2024. Front Immunol. 2024. PMID: 38881902 Free PMC article. Review.
References
-
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–198. doi: 10.1158/2159-8290.Cd-18-1177. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

