Preparation and Characterization Studies of Dorzolamide-Loaded Ophthalmic Implants for Treating Glaucoma
- PMID: 37417193
- PMCID: PMC10337019
- DOI: 10.4274/tjps.galenos.2022.95752
Preparation and Characterization Studies of Dorzolamide-Loaded Ophthalmic Implants for Treating Glaucoma
Abstract
Objectives: This study constructed dorzolamide (DRZ)-loaded ophthalmic implants for extended drug delivery and increased drug retention.
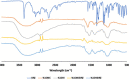
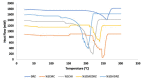
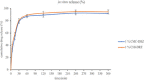
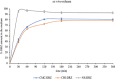
Materials and methods: Carboxymethyl cellulose (CMC) and chitosan (CHI) were used to describe the ophthalmic implants. The implants were prepared by the solvent casting technique in presence of polyethylene glycol 6000 (PEG 6000) as plasticizer. Physicochemical characterization studies including mechanical characteristics [tensile strength (TS), elongation at break, and Young's modulus], bioadhesion studies, and in vitro and ex vivo drug release studies were conducted.
Results: TS of drug-loaded ophthalmic implants was 10.70 and 11.68 MPa, respectively. Elongation at break of CMC and CHI implants was 62.00% and 59.05%, respectively. The in vitro release profiles fit into Higuchi type kinetic model. Ex vivo release study results for both implants were correlated with in vitro release investigations.
Conclusion: CMC and CHI-based implants provide extended drug delivery. Implants prepared using CMC provided a significantly slower in vitro release rate, and drug retention on ocular surfaces increased. Thus, it has been concluded that DRZ-loaded CMC implants could provide effective treatment for glaucoma.
Keywords: Dorzolamide; carboxymethyl cellulose; chitosan; ocular implant.
©Copyright 2023 by Turkish Pharmacists' Association / Turkish Journal of Pharmaceutical Sciences published by Galenos Publishing House.
Conflict of interest statement
Conflict of Interest: No conflict of interest was declared by the authors.
Figures

Similar articles
-
Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation.Int J Pharm. 2019 Oct 30;570:118662. doi: 10.1016/j.ijpharm.2019.118662. Epub 2019 Sep 3. Int J Pharm. 2019. PMID: 31491481
-
Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments.Int J Biol Macromol. 2020 Nov 15;163:2392-2404. doi: 10.1016/j.ijbiomac.2020.09.185. Epub 2020 Sep 23. Int J Biol Macromol. 2020. PMID: 32979440
-
Preparation and characterization of carboxymethyl cellulose containing quaternized chitosan for potential drug carrier.Int J Biol Macromol. 2020 Jul 1;154:1392-1399. doi: 10.1016/j.ijbiomac.2019.11.019. Epub 2019 Nov 12. Int J Biol Macromol. 2020. PMID: 31730962
-
Ocular Dorzolamide Nanoliposomes for Prolonged IOP Reduction: in-vitroand in-vivo Evaluation in Rabbits.Iran J Pharm Res. 2016 Winter;15(1):205-12. Iran J Pharm Res. 2016. PMID: 27610160 Free PMC article.
-
Designing of a pH-Triggered Carbopol®/HPMC In Situ Gel for Ocular Delivery of Dorzolamide HCl: In Vitro, In Vivo, and Ex Vivo Evaluation.AAPS PharmSciTech. 2019 Jun 3;20(5):210. doi: 10.1208/s12249-019-1431-y. AAPS PharmSciTech. 2019. PMID: 31161269
Cited by
-
Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases.Pharmaceutics. 2023 Jun 15;15(6):1746. doi: 10.3390/pharmaceutics15061746. Pharmaceutics. 2023. PMID: 37376194 Free PMC article. Review.
References
-
- Lang GK. Ophthalmology: a short textbook. New York; Thieme Medical Publishers. 2000.
-
- Abel SR, Sorensen SJ. Eye disorders. In: Koda-Kimble MA, eds., Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs. Wolters Kluwer Health/Lippincott Williams & Wilkins. 2012:1301–1322.
-
- Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem. 2014;75:349–359. - PubMed
-
- Donohue EK, Wilensky JT. Trusopt, a topical carbonic anhydrase inhibitor. J Glaucoma. 1996;5:68–74. - PubMed
-
- Suri R, Beg S, Kohli K. Target strategies for drug delivery bypassing ocular barriers. J Drug Deliv Sci Technol. 2020;55:101389.
LinkOut - more resources
Full Text Sources
