Improving dental epithelial junction on dental implants with bioengineered peptides
- PMID: 37409165
- PMCID: PMC10318435
- DOI: 10.3389/fbioe.2023.1165853
Improving dental epithelial junction on dental implants with bioengineered peptides
Abstract
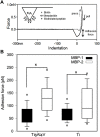
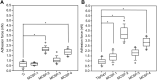
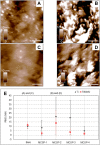
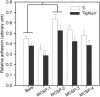
Introduction: The functionalization of titanium (Ti) and titanium alloys (Ti6Al4V) implant surfaces via material-specific peptides influence host/biomaterial interaction. The impact of using peptides as molecular linkers between cells and implant material to improve keratinocyte adhesion is reported. Results: The metal binding peptides (MBP-1, MBP-2) SVSVGMKPSPRP and WDPPTLKRPVSP were selected via phage display and combined with laminin-5 or E-cadherin epithelial cell specific peptides (CSP-1, CSP-2) to engineer four metal-cell specific peptides (MCSPs). Single-cell force spectroscopy and cell adhesion experiments were performed to select the most promising candidate. In vivo tests using the dental implant for rats showed that the selected bi functional peptide not only enabled stable cell adhesion on the trans-gingival part of the dental implant but also arrested the unwanted apical migration of epithelial cells. Conclusion: The results demonstrated the outstanding performance of the bioengineered peptide in improving epithelial adhesion to Ti based implants and pointed towards promising new opportunities for applications in clinical practice.
Keywords: bioengineered peptide; epithelial adhesion; implants; phage display; titanium surface functionalization.
Copyright © 2023 Panayotov, Végh, Martin, Vladimirov, Larroque, Gergely, Cuisinier and Estephan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implants.Biomaterials. 2009 Apr;30(12):2291-301. doi: 10.1016/j.biomaterials.2009.01.004. Epub 2009 Jan 24. Biomaterials. 2009. PMID: 19168216
-
Biological response on a titanium implant-grade surface functionalized with modular peptides.Acta Biomater. 2013 Feb;9(2):5341-52. doi: 10.1016/j.actbio.2012.11.004. Epub 2012 Nov 14. Acta Biomater. 2013. PMID: 23159566 Free PMC article.
-
Biocompatibility of Subperiosteal Dental Implants: Effects of Differently Treated Titanium Surfaces on the Expression of ECM-Related Genes in Gingival Fibroblasts.J Funct Biomater. 2023 Jan 20;14(2):59. doi: 10.3390/jfb14020059. J Funct Biomater. 2023. PMID: 36826858 Free PMC article.
-
Strategies For Immobilization Of Bioactive Organic Molecules On Titanium Implant Surfaces - A Review.Folia Med (Plovdiv). 2015 Jan-Mar;57(1):11-8. doi: 10.1515/folmed-2015-0014. Folia Med (Plovdiv). 2015. PMID: 26431090 Review.
-
Preventing Peri-implantitis: The Quest for a Next Generation of Titanium Dental Implants.ACS Biomater Sci Eng. 2022 Nov 14;8(11):4697-4737. doi: 10.1021/acsbiomaterials.2c00540. Epub 2022 Oct 14. ACS Biomater Sci Eng. 2022. PMID: 36240391 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

