The Role of FBXW7 in Gynecologic Malignancies
- PMID: 37408248
- PMCID: PMC10216672
- DOI: 10.3390/cells12101415
The Role of FBXW7 in Gynecologic Malignancies
Abstract
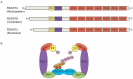
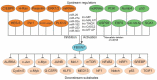
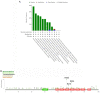
The F-Box and WD Repeat Domain Containing 7 (FBXW7) protein has been shown to regulate cellular growth and act as a tumor suppressor. This protein, also known as FBW7, hCDC4, SEL10 or hAGO, is encoded by the gene FBXW7. It is a crucial component of the Skp1-Cullin1-F-box (SCF) complex, which is a ubiquitin ligase. This complex aids in the degradation of many oncoproteins, such as cyclin E, c-JUN, c-MYC, NOTCH, and MCL1, via the ubiquitin-proteasome system (UPS). The FBXW7 gene is commonly mutated or deleted in numerous types of cancer, including gynecologic cancers (GCs). Such FBXW7 mutations are linked to a poor prognosis due to increased treatment resistance. Hence, detection of the FBXW7 mutation may possibly be an appropriate diagnostic and prognostic biomarker that plays a central role in determining suitable individualized management. Recent studies also suggest that, under specific circumstances, FBXW7 may act as an oncogene. There is mounting evidence indicating that the aberrant expression of FBXW7 is involved in the development of GCs. The aim of this review is to give an update on the role of FBXW7 as a potential biomarker and also as a therapeutic target for novel treatments, particularly in the management of GCs.
Keywords: FBXW7; LncRNAs; epigenetic; gynecologic cancers; miRNAs; mutations; target therapy; ubiquitin-proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Recent insight into the role of FBXW7 as a tumor suppressor.Semin Cancer Biol. 2020 Dec;67(Pt 2):1-15. doi: 10.1016/j.semcancer.2020.02.017. Epub 2020 Feb 27. Semin Cancer Biol. 2020. PMID: 32113998 Review.
-
FBXW7: a critical tumor suppressor of human cancers.Mol Cancer. 2018 Aug 7;17(1):115. doi: 10.1186/s12943-018-0857-2. Mol Cancer. 2018. PMID: 30086763 Free PMC article. Review.
-
The FBXW7-NOTCH interactome: A ubiquitin proteasomal system-induced crosstalk modulating oncogenic transformation in human tissues.Cancer Rep (Hoboken). 2021 Aug;4(4):e1369. doi: 10.1002/cnr2.1369. Epub 2021 Apr 6. Cancer Rep (Hoboken). 2021. PMID: 33822486 Free PMC article. Review.
-
Recombinant human adenovirus-p53 injection induced apoptosis in hepatocellular carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls c-Myc and cyclin E.PLoS One. 2013 Jul 1;8(7):e68574. doi: 10.1371/journal.pone.0068574. Print 2013. PLoS One. 2013. Retraction in: PLoS One. 2020 Mar 27;15(3):e0231287. doi: 10.1371/journal.pone.0231287 PMID: 23840897 Free PMC article. Retracted.
-
Fbxw7 contributes to tumor suppression by targeting multiple proteins for ubiquitin-dependent degradation.Cancer Sci. 2006 Aug;97(8):729-36. doi: 10.1111/j.1349-7006.2006.00239.x. Cancer Sci. 2006. PMID: 16863506 Free PMC article.
Cited by
-
Comparative analysis of EZH2, p16 and p53 expression in uterine carcinosarcomas.Pathol Oncol Res. 2023 Dec 11;29:1611547. doi: 10.3389/pore.2023.1611547. eCollection 2023. Pathol Oncol Res. 2023. PMID: 38146588 Free PMC article.
-
Targeted and Shallow Whole-Genome Sequencing Identifies Therapeutic Opportunities in p53abn Endometrial Cancers.Clin Cancer Res. 2024 Jun 3;30(11):2461-2474. doi: 10.1158/1078-0432.CCR-23-3689. Clin Cancer Res. 2024. PMID: 38536067 Free PMC article.
-
Targeted variant prevalence of FBXW7 gene mutation in colorectal carcinoma propagation. The first systematic review and meta-analysis.Heliyon. 2024 May 22;10(11):e31471. doi: 10.1016/j.heliyon.2024.e31471. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38845996 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

