Metabolo-epigenetic interplay provides targeted nutritional interventions in chronic diseases and ageing
- PMID: 37404756
- PMCID: PMC10315663
- DOI: 10.3389/fonc.2023.1169168
Metabolo-epigenetic interplay provides targeted nutritional interventions in chronic diseases and ageing
Abstract
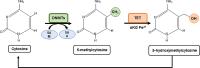
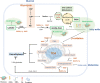
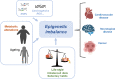
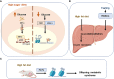
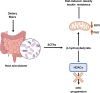
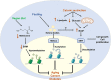
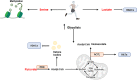
Epigenetic modifications are chemical modifications that affect gene expression without altering DNA sequences. In particular, epigenetic chemical modifications can occur on histone proteins -mainly acetylation, methylation-, and on DNA and RNA molecules -mainly methylation-. Additional mechanisms, such as RNA-mediated regulation of gene expression and determinants of the genomic architecture can also affect gene expression. Importantly, depending on the cellular context and environment, epigenetic processes can drive developmental programs as well as functional plasticity. However, misbalanced epigenetic regulation can result in disease, particularly in the context of metabolic diseases, cancer, and ageing. Non-communicable chronic diseases (NCCD) and ageing share common features including altered metabolism, systemic meta-inflammation, dysfunctional immune system responses, and oxidative stress, among others. In this scenario, unbalanced diets, such as high sugar and high saturated fatty acids consumption, together with sedentary habits, are risk factors implicated in the development of NCCD and premature ageing. The nutritional and metabolic status of individuals interact with epigenetics at different levels. Thus, it is crucial to understand how we can modulate epigenetic marks through both lifestyle habits and targeted clinical interventions -including fasting mimicking diets, nutraceuticals, and bioactive compounds- which will contribute to restore the metabolic homeostasis in NCCD. Here, we first describe key metabolites from cellular metabolic pathways used as substrates to "write" the epigenetic marks; and cofactors that modulate the activity of the epigenetic enzymes; then, we briefly show how metabolic and epigenetic imbalances may result in disease; and, finally, we show several examples of nutritional interventions - diet based interventions, bioactive compounds, and nutraceuticals- and exercise to counteract epigenetic alterations.
Keywords: ageing; bioactive compounds; chronic diseases; epigenetics; metabolism.
Copyright © 2023 Gómez de Cedrón, Moreno Palomares and Ramírez de Molina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Interplay Among Metabolism, Epigenetic Modifications, and Gene Expression in Cancer.Front Cell Dev Biol. 2021 Dec 24;9:793428. doi: 10.3389/fcell.2021.793428. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 35004688 Free PMC article. Review.
-
Present and future of anti-ageing epigenetic diets.Mech Ageing Dev. 2014 Mar-Apr;136-137:101-15. doi: 10.1016/j.mad.2013.12.006. Epub 2014 Jan 2. Mech Ageing Dev. 2014. PMID: 24388875 Review.
-
Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives.Mol Aspects Med. 2013 Jul-Aug;34(4):782-812. doi: 10.1016/j.mam.2012.06.010. Epub 2012 Jul 4. Mol Aspects Med. 2013. PMID: 22771541 Review.
-
Nutritional developmental epigenomics: immediate and long-lasting effects.Proc Nutr Soc. 2010 May;69(2):221-31. doi: 10.1017/S002966511000008X. Epub 2010 Mar 5. Proc Nutr Soc. 2010. PMID: 20202279
Cited by
-
Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies.J Clin Med. 2024 Mar 20;13(6):1774. doi: 10.3390/jcm13061774. J Clin Med. 2024. PMID: 38541997 Free PMC article. Review.
-
Nutrigenomics and redox regulation: Concepts relating to the Special Issue on nutrigenomics.Redox Biol. 2023 Dec;68:102920. doi: 10.1016/j.redox.2023.102920. Epub 2023 Oct 4. Redox Biol. 2023. PMID: 37839954 Free PMC article. Review.
-
Circular RNAs, Noncoding RNAs, and N6-methyladenosine Involved in the Development of MAFLD.Noncoding RNA. 2024 Feb 5;10(1):11. doi: 10.3390/ncrna10010011. Noncoding RNA. 2024. PMID: 38392966 Free PMC article. Review.
References
-
- Global Burden of Disease Collaborative Network. G. B. o. D. S. G. R. Institute for Health Metrics and Evaluation - IHME . .
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

