Ablation of the deubiquitinase USP15 ameliorates nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
- PMID: 37394587
- PMCID: PMC10394025
- DOI: 10.1038/s12276-023-01036-7
Ablation of the deubiquitinase USP15 ameliorates nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
Erratum in
-
Author Correction: Ablation of the deubiquitinase USP15 ameliorates nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.Exp Mol Med. 2024 Oct 28. doi: 10.1038/s12276-024-01343-7. Online ahead of print. Exp Mol Med. 2024. PMID: 39468328 No abstract available.
Abstract
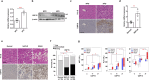
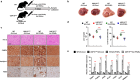
Nonalcoholic fatty liver disease (NAFLD) occurs due to the accumulation of fat in the liver, leading to fatal liver diseases such as nonalcoholic steatohepatitis (NASH) and cirrhosis. Elucidation of the molecular mechanisms underlying NAFLD is critical for its prevention and therapy. Here, we observed that deubiquitinase USP15 expression was upregulated in the livers of mice fed a high-fat diet (HFD) and liver biopsies of patients with NAFLD or NASH. USP15 interacts with lipid-accumulating proteins such as FABPs and perilipins to reduce ubiquitination and increase their protein stability. Furthermore, the severity of NAFLD induced by an HFD and NASH induced by a fructose/palmitate/cholesterol/trans-fat (FPC) diet was significantly ameliorated in hepatocyte-specific USP15 knockout mice. Thus, our findings reveal an unrecognized function of USP15 in the lipid accumulation of livers, which exacerbates NAFLD to NASH by overriding nutrients and inducing inflammation. Therefore, targeting USP15 can be used in the prevention and treatment of NAFLD and NASH.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Heterozygous midnolin knockout attenuates severity of nonalcoholic fatty liver disease in mice fed a Western-style diet high in fat, cholesterol, and fructose.Am J Physiol Gastrointest Liver Physiol. 2023 Aug 1;325(2):G147-G157. doi: 10.1152/ajpgi.00011.2023. Epub 2023 May 2. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 37129245 Free PMC article.
-
The American lifestyle-induced obesity syndrome diet in male and female rodents recapitulates the clinical and transcriptomic features of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.Am J Physiol Gastrointest Liver Physiol. 2020 Sep 1;319(3):G345-G360. doi: 10.1152/ajpgi.00055.2020. Epub 2020 Aug 5. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32755310 Free PMC article.
-
Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder.Theranostics. 2020 Aug 1;10(21):9702-9720. doi: 10.7150/thno.44297. eCollection 2020. Theranostics. 2020. PMID: 32863955 Free PMC article.
-
A Comparison of the Gene Expression Profiles of Non-Alcoholic Fatty Liver Disease between Animal Models of a High-Fat Diet and Methionine-Choline-Deficient Diet.Molecules. 2022 Jan 27;27(3):858. doi: 10.3390/molecules27030858. Molecules. 2022. PMID: 35164140 Free PMC article. Review.
-
Potential Therapeutic Application of Estrogen in Gender Disparity of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis.Cells. 2019 Oct 15;8(10):1259. doi: 10.3390/cells8101259. Cells. 2019. PMID: 31619023 Free PMC article. Review.
Cited by
-
Deubiquitinase USP1 enhances CCAAT/enhancer-binding protein beta (C/EBPβ) stability and accelerates adipogenesis and lipid accumulation.Cell Death Dis. 2023 Nov 27;14(11):776. doi: 10.1038/s41419-023-06317-7. Cell Death Dis. 2023. PMID: 38012162 Free PMC article.
References
-
- Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol.62, S47–S64 (2015). - PubMed
-
- Michelotti, G. A., Machado, M. V. & Diehl, A. M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol.10, 656–665 (2013). - PubMed
-
- Eslam, M., Valenti, L. & Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol.68, 268–279 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

