Flow cytometric method for the detection and quantification of retinal cell death and oxidative stress
- PMID: 37393050
- PMCID: PMC10794879
- DOI: 10.1016/j.exer.2023.109563
Flow cytometric method for the detection and quantification of retinal cell death and oxidative stress
Abstract
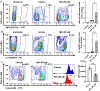
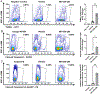
Retinal cell death is the major cause of vision loss in many forms of blinding retinal disease. A plethora of research is focused on understanding the mechanisms of retinal cell death to identify potential neuroprotective strategies that prevent vision loss in these diseases. Traditionally, histological techniques have been used to determine the type and extent of cell death in the retina. These techniques, such as TUNEL labeling and immunohistochemistry, are laborious and time consuming, resulting in low throughput and variable results depending on the experimenter. To increase throughput and reduce variability, we developed several flow cytometry-based assays to detect and quantify retinal cell death. The methods and accompanying data presented demonstrate that flow cytometry can readily detect both retinal cell death and oxidative stress and importantly, the efficacy of neuroprotective agents. These methods will be of interest to investigators looking to increase throughput and efficiency without compromising sensitivity as the methods herein reduce analysis time from several months to less than a week. As such, the flow cytometry methods presented have the potential to expedite research efforts focused on developing novel strategies for retinal cell neuroprotection.
Keywords: Apoptosis; Caspase; Flow cytometry; Reactive oxygen species; Retina; Retinal detachment.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Protective effect of molecular hydrogen against oxidative stress caused by peroxynitrite derived from nitric oxide in rat retina.Clin Exp Ophthalmol. 2015 Aug;43(6):568-77. doi: 10.1111/ceo.12525. Epub 2015 May 13. Clin Exp Ophthalmol. 2015. PMID: 25801048
-
Neuroprotective Effect of Magnesium Acetyltaurate Against NMDA-Induced Excitotoxicity in Rat Retina.Neurotox Res. 2017 Jan;31(1):31-45. doi: 10.1007/s12640-016-9658-9. Epub 2016 Aug 27. Neurotox Res. 2017. PMID: 27568334
-
Cell Death Analysis in Retinal Cultures.Methods Mol Biol. 2019;1834:143-152. doi: 10.1007/978-1-4939-8669-9_10. Methods Mol Biol. 2019. PMID: 30324442
-
[Comprehensive strategy for retinal neuroprotection. Challenging the clinical application].Nippon Ganka Gakkai Zasshi. 2012 Mar;116(3):165-98; discussion 199. Nippon Ganka Gakkai Zasshi. 2012. PMID: 22568101 Review. Japanese.
-
Molecular mechanisms of neuroprotection in the eye.Adv Exp Med Biol. 2006;572:291-5. doi: 10.1007/0-387-32442-9_40. Adv Exp Med Biol. 2006. PMID: 17249586 Review. No abstract available.
Cited by
-
Metabolic Alterations Caused by Simultaneous Loss of HK2 and PKM2 Leads to Photoreceptor Dysfunction and Degeneration.Cells. 2023 Aug 10;12(16):2043. doi: 10.3390/cells12162043. Cells. 2023. PMID: 37626853 Free PMC article.
References
-
- Awwad S, Henein C, Ibeanu N, Khaw PT, Brocchini S, 2020. Preclinical challenges for developing long acting intravitreal medicines. Eur. J. Pharm. Biopharm 153, 130–149. - PubMed
-
- Carmody RJ, McGowan AJ, Cotter TG, 1998. Rapid detection of rod photoreceptor apoptosis by flow cytometry. Cytometry 33, 89–92. - PubMed
-
- Chan TC, Wilkinson Berka JL, Deliyanti D, Hunter D, Fung A, Liew G, White A, 2020. The role of reactive oxygen species in the pathogenesis and treatment of retinal diseases. Exp. Eye Res 201, 108255. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

