Ex vivo ocular perfusion model to study vascular physiology in the mouse eye
- PMID: 37390954
- PMCID: PMC10637262
- DOI: 10.1016/j.exer.2023.109543
Ex vivo ocular perfusion model to study vascular physiology in the mouse eye
Abstract
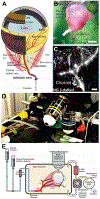
Several hypotheses have been tested to understand whole organ regulation in other organs such as the brain and kidney, but no such hypothesis has yet been proposed for ocular circulations. To some extent resolve this deficit our ex vivo mouse eye perfusion model takes the first step in elucidating the mechanisms controlling the individual components of the ocular circulation. Various isolated ocular vascular preparations have been utilized in studies of ocular vascular biology, physiology, and pharmacology, including studies on both normal and pathological conditions. However, there is still significant potential for further studies to improve our understanding of ocular circulation and its regulation. The choroid specifically is inaccessible to direct visualization due to the retina's high metabolic requirement with a transparency that cannot be compromised by an overly rich vascular network on the inner retinal side hindering the visualization of the choroid. In this technical paper, we provide a detailed description of all the steps to be followed from the enucleation of mouse eyes to cannulation of the ophthalmic artery and perfusion and ex vivo confocal microscopy imaging of the dynamic nature of the choroid circulation.
Published by Elsevier Ltd.
Figures



Similar articles
-
Isolated preparations of ocular vasculature and their applications in ophthalmic research.Prog Retin Eye Res. 2003 Mar;22(2):135-69. doi: 10.1016/s1350-9462(02)00044-7. Prog Retin Eye Res. 2003. PMID: 12604056 Review.
-
A technical protocol for an experimental ex vivo model using arterially perfused porcine eyes.Exp Eye Res. 2019 Apr;181:171-177. doi: 10.1016/j.exer.2019.02.003. Epub 2019 Feb 5. Exp Eye Res. 2019. PMID: 30735657
-
Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration.Mol Vis. 2008 Jan 29;14:150-60. Mol Vis. 2008. PMID: 18334929 Free PMC article.
-
[In vivo measurement of ocular circulation with the laser speckle method--development of apparatus and application in ophthalmological research].Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):871-909. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643292 Review. Japanese.
-
Optical imaging of the chorioretinal vasculature in the living human eye.Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14354-9. doi: 10.1073/pnas.1307315110. Epub 2013 Aug 5. Proc Natl Acad Sci U S A. 2013. PMID: 23918361 Free PMC article.
Cited by
-
Light-sensitive Ca2+ signaling in the mammalian choroid.Proc Natl Acad Sci U S A. 2024 Nov 12;121(46):e2418429121. doi: 10.1073/pnas.2418429121. Epub 2024 Nov 8. Proc Natl Acad Sci U S A. 2024. PMID: 39514305 Free PMC article.
References
-
- Alm A, Bill A, 1970. Blood Flow and Oxygen Extraction in the Cat Uvea at Normal and High Intraocular Pressures. Acta Physiologica Scandinavica 80, 19–28. - PubMed
-
- Chang B, Hawes NL, Hurd RE, Wang J, Howell D, Davisson MT, Roderick TH, Nusinowitz S, Heckenlively JR, 2005. Mouse models of ocular diseases. Visual Neuroscience 22, 587–593. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

