Development potential of extracellular matrix hydrogels as hemostatic materials
- PMID: 37383519
- PMCID: PMC10294235
- DOI: 10.3389/fbioe.2023.1187474
Development potential of extracellular matrix hydrogels as hemostatic materials
Abstract
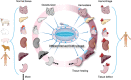
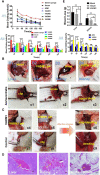
The entry of subcutaneous extracellular matrix proteins into the circulation is a key step in hemostasis initiation after vascular injury. However, in cases of severe trauma, extracellular matrix proteins are unable to cover the wound, making it difficult to effectively initiate hemostasis and resulting in a series of bleeding events. Acellular-treated extracellular matrix (ECM) hydrogels are widely used in regenerative medicine and can effectively promote tissue repair due to their high mimic nature and excellent biocompatibility. ECM hydrogels contain high concentrations of extracellular matrix proteins, including collagen, fibronectin, and laminin, which can simulate subcutaneous extracellular matrix components and participate in the hemostatic process. Therefore, it has unique advantages as a hemostatic material. This paper first reviewed the preparation, composition and structure of extracellular hydrogels, as well as their mechanical properties and safety, and then analyzed the hemostatic mechanism of the hydrogels to provide a reference for the application and research, and development of ECM hydrogels in the field of hemostasis.
Keywords: extracellular matrix components (ECM); extracellular matrix hydrogel; hemorrhage; hemostasis; hemostatic mechanism.
Copyright © 2023 Cai and Weng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Preparation and Application of Hemostatic Hydrogels.Small. 2024 May;20(22):e2309485. doi: 10.1002/smll.202309485. Epub 2023 Dec 15. Small. 2024. PMID: 38102098 Review.
-
Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing.Adv Healthc Mater. 2020 Oct;9(20):e2000905. doi: 10.1002/adhm.202000905. Epub 2020 Sep 16. Adv Healthc Mater. 2020. PMID: 32940025 Review.
-
Design of Advanced Polymeric Hydrogels for Tissue Regenerative Medicine: Oxygen-Controllable Hydrogel Materials.Adv Exp Med Biol. 2020;1250:63-78. doi: 10.1007/978-981-15-3262-7_5. Adv Exp Med Biol. 2020. PMID: 32601938 Review.
-
Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects.Acta Biomater. 2018 Jun;73:326-338. doi: 10.1016/j.actbio.2018.04.001. Epub 2018 Apr 9. Acta Biomater. 2018. PMID: 29649641
-
Intact vitreous humor as a potential extracellular matrix hydrogel for cartilage tissue engineering applications.Acta Biomater. 2019 Feb;85:117-130. doi: 10.1016/j.actbio.2018.12.022. Epub 2018 Dec 18. Acta Biomater. 2019. PMID: 30572166
Cited by
-
Non-Covalent Cross-Linking Hydrogel: A New Method for Visceral Hemostasis.Gels. 2024 May 10;10(5):326. doi: 10.3390/gels10050326. Gels. 2024. PMID: 38786243 Free PMC article.
-
Mimicking Molecular Pathways in the Design of Smart Hydrogels for the Design of Vascularized Engineered Tissues.Int J Mol Sci. 2023 Aug 1;24(15):12314. doi: 10.3390/ijms241512314. Int J Mol Sci. 2023. PMID: 37569691 Free PMC article. Review.
References
-
- Adam Young D., Bajaj V., Christman K. L. (2014). Award winner for outstanding research in the PhD category, 2014 Society for Biomaterials annual meeting and exposition, Denver, Colorado, April 16-19, 2014: Decellularized adipose matrix hydrogels stimulate in vivo neovascularization and adipose formation. J. Biomed. Mater. Res. Part A. 102 (6), 1641–1651. 10.1002/jbm.a.35109 - DOI - PubMed
-
- Attwood S. J., Simpson A. M., Hamaia S. W., Bihan D., Roy D., Farndale R. W., et al. (2013). Measurement of the interaction between recombinant I-domain from integrin alpha 2 beta 1 and a triple helical collagen peptide with the GFOGER binding motif using molecular force spectroscopy. Int. J. Mol. Sci. 14 (2), 2832–2845. 10.3390/ijms14022832 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

