Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial
- PMID: 37373762
- PMCID: PMC10299137
- DOI: 10.3390/jcm12124069
Anterior Cervical Discectomy and Fusion Performed Using a CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic or Polyetheretherketone Cage Filled with Hydroxyapatite/β-Tricalcium Phosphate: A Prospective Randomized Controlled Trial
Abstract
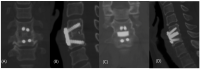
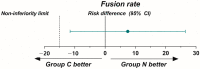
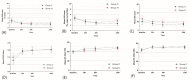
A CaO-SiO2-P2O5-B2O3 bioactive glass-ceramic (BGS-7) spacer provides high mechanical stability, produces a chemical bond to the adjacent endplate, and facilitates fusion after spine surgery. This prospective, randomized, single-blind, non-inferiority trial aimed to evaluate the radiographic outcomes and clinical efficacy of anterior cervical discectomy and fusion (ACDF) using a BGS-7 spacer for treating cervical degenerative disorders. Thirty-six patients underwent ACDF using a BGS-7 spacer (Group N), and 40 patients underwent ACDF using polyetheretherketone (PEEK) cages filled with a mixture of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) for the treatment of cervical degenerative disorders. The spinal fusion rate was assessed 12 months postoperatively using three-dimensional computed tomography (CT) and dynamic radiographs. Clinical outcomes included patient-reported outcome measures, visual analog scale scores for neck and arm pain, and scores from the neck disability index (NDI), European Quality of Life-5 Dimensions (EQ-5D), and 12-item Short Form Survey (SF-12v2). All participants were randomly assigned to undergo ACDF using either a BGS-7 spacer or PEEK cage filled with HA and β-TCP. The primary outcome was the fusion rate on CT scan image at 12 months after ACDF surgery based on a per-protocol strategy. Clinical outcomes and adverse events were also assessed. The 12-month fusion rates for the BGS-7 and PEEK groups based on CT scans were 81.8% and 74.4%, respectively, while those based on dynamic radiographs were 78.1% and 73.7%, respectively, with no significant difference between the groups. There were no significant differences in the clinical outcomes between the two groups. Neck pain, arm pain, NDI, EQ-5D, and SF-12v2 scores significantly improved postoperatively, with no significant differences between the groups. No adverse events were observed in either group. In ACDF surgery, the BGS-7 spacer showed similar fusion rates and clinical outcomes as PEEK cages filled with HA and β-TCP.
Keywords: ACDF; PEEK cage; cervical; fusion rate; glass ceramics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Clinical and radiological outcomes of non-window-type bioactive glass-ceramic cage in single-level ACDF versus PEEK cage filled with autologous bone.Sci Rep. 2024 Feb 19;14(1):4035. doi: 10.1038/s41598-024-54786-3. Sci Rep. 2024. PMID: 38369553 Free PMC article.
-
Feasibility and safety of a CaO-SiO2-P2O5-B2O3 bioactive glass ceramic spacer in posterior lumbar interbody fusion compared with polyetheretherketone cage: a prospective randomized controlled trial.Acta Neurochir (Wien). 2023 Jan;165(1):135-144. doi: 10.1007/s00701-022-05429-x. Epub 2022 Dec 6. Acta Neurochir (Wien). 2023. PMID: 36471204 Clinical Trial.
-
Feasibility of CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramic Cage in Anterior Cervical Diskectomy and Fusion.World Neurosurg. 2020 Sep;141:e358-e366. doi: 10.1016/j.wneu.2020.05.143. Epub 2020 May 22. World Neurosurg. 2020. PMID: 32450308 Clinical Trial.
-
Review of anterior cervical diskectomy/fusion (ACDF) using different polyetheretherketone (PEEK) cages.Surg Neurol Int. 2022 Nov 25;13:556. doi: 10.25259/SNI_992_2022. eCollection 2022. Surg Neurol Int. 2022. PMID: 36600749 Free PMC article. Review.
-
Safety and effectiveness of bone allografts in anterior cervical discectomy and fusion surgery.Spine (Phila Pa 1976). 2011 Nov 15;36(24):2045-50. doi: 10.1097/BRS.0b013e3181ff37eb. Spine (Phila Pa 1976). 2011. PMID: 21304437 Review.
Cited by
-
Clinical and radiological outcomes of non-window-type bioactive glass-ceramic cage in single-level ACDF versus PEEK cage filled with autologous bone.Sci Rep. 2024 Feb 19;14(1):4035. doi: 10.1038/s41598-024-54786-3. Sci Rep. 2024. PMID: 38369553 Free PMC article.
-
Current and Future Perspectives of Bioactive Glasses as Injectable Material.Nanomaterials (Basel). 2024 Jul 13;14(14):1196. doi: 10.3390/nano14141196. Nanomaterials (Basel). 2024. PMID: 39057873 Free PMC article. Review.
References
-
- D’Antonio N., Lambrechts M.J., Heard J., Bertiaume E., Toci G., Karamian B., Breyer G., Bodnar J., Canseco J., Hilibrand A. Effect of Interbody Composition on the Development of Pseudarthrosis Following Anterior Cervical Discectomy and Fusion. Asian Spine J. 2023 doi: 10.31616/asj.2022.0258. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

