Differentiation Capacity of Porcine Skeletal Muscle-Derived Stem Cells as Intermediate Species between Mice and Humans
- PMID: 37373009
- PMCID: PMC10297882
- DOI: 10.3390/ijms24129862
Differentiation Capacity of Porcine Skeletal Muscle-Derived Stem Cells as Intermediate Species between Mice and Humans
Abstract
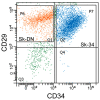
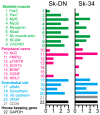
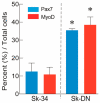
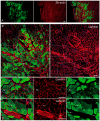
Large animal experiments are important for preclinical studies of regenerative stem cell transplantation therapy. Therefore, we investigated the differentiation capacity of pig skeletal muscle-derived stem cells (Sk-MSCs) as an intermediate model between mice and humans for nerve muscle regenerative therapy. Enzymatically extracted cells were obtained from green-fluorescence transgenic micro-mini pigs (GFP-Tg MMP) and sorted as CD34+/45- (Sk-34) and CD34-/45-/29+ (Sk-DN) fractions. The ability to differentiate into skeletal muscle, peripheral nerve, and vascular cell lineages was examined via in vitro cell culture and in vivo cell transplantation into the damaged tibialis anterior muscle and sciatic nerves of nude mice and rats. Protein and mRNA levels were analyzed using RT-PCR, immunohistochemistry, and immunoelectron microscopy. The myogenic potential, which was tested by Pax7 and MyoD expression and the formation of muscle fibers, was higher in Sk-DN cells than in Sk-34 cells but remained weak in the latter. In contrast, the capacity to differentiate into peripheral nerve and vascular cell lineages was significantly stronger in Sk-34 cells. In particular, Sk-DN cells did not engraft to the damaged nerve, whereas Sk-34 cells showed active engraftment and differentiation into perineurial/endoneurial cells, endothelial cells, and vascular smooth muscle cells, similar to the human case, as previously reported. Therefore, we concluded that Sk-34 and Sk-DN cells in pigs are closer to those in humans than to those in mice.
Keywords: GFP-transgenic pig; large animal experiment; micro-mini pig; multipotent stem cells; nerve-muscle regeneration; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synchronized reconstitution of muscle fibers, peripheral nerves and blood vessels by murine skeletal muscle-derived CD34(-)/45 (-) cells.Histochem Cell Biol. 2007 Oct;128(4):349-60. doi: 10.1007/s00418-007-0331-5. Epub 2007 Aug 29. Histochem Cell Biol. 2007. PMID: 17762938
-
A Long-Gap Peripheral Nerve Injury Therapy Using Human Skeletal Muscle-Derived Stem Cells (Sk-SCs): An Achievement of Significant Morphological, Numerical and Functional Recovery.PLoS One. 2016 Nov 15;11(11):e0166639. doi: 10.1371/journal.pone.0166639. eCollection 2016. PLoS One. 2016. PMID: 27846318 Free PMC article.
-
Skeletal muscle-derived CD34+/45- and CD34-/45- stem cells are situated hierarchically upstream of Pax7+ cells.Stem Cells Dev. 2008 Aug;17(4):653-67. doi: 10.1089/scd.2008.0070. Stem Cells Dev. 2008. PMID: 18554087
-
Plasticity and physiological role of stem cells derived from skeletal muscle interstitium: contribution to muscle fiber hyperplasia and therapeutic use.Curr Pharm Des. 2010;16(8):956-67. doi: 10.2174/138161210790883408. Curr Pharm Des. 2010. PMID: 20041822 Review.
-
Bridging long gap peripheral nerve injury using skeletal muscle-derived multipotent stem cells.Neural Regen Res. 2014 Jul 15;9(14):1333-6. doi: 10.4103/1673-5374.137582. Neural Regen Res. 2014. PMID: 25221587 Free PMC article. Review.
Cited by
-
Skeletal Muscle-Derived Stem Cell Transplantation Accelerates the Recovery of Peripheral Nerve Gap Injury under 50% and 100% Allogeneic Compatibility with the Swine Leucocyte Antigen.Biomolecules. 2024 Aug 2;14(8):939. doi: 10.3390/biom14080939. Biomolecules. 2024. PMID: 39199327 Free PMC article.
References
-
- Kawaguchi H., Miyoshi N., Miura N., Fujiki M., Horiuchi M., Izumi Y., Miyajima H., Nagata R., Misumi K., Takeuchi T., et al. Microminipig, a non-rodent experimental animal optimized for life science research: Novel atherosclerosis model induced by high fat and cholesterol diet. J. Pharmacol. Sci. 2011;115:115–121. doi: 10.1254/jphs.10R17FM. - DOI - PubMed
-
- Sugiyama A., Nakamura Y., Akie Y., Saito H., Izumi Y., Yamazaki H., Kaneko N., Itoh K. Microminipig, a non-rodent experimental animal optimized for life science research: In vivo proarrhythmia models of drug-induced long qt syndrome: Development of chronic atrioventricular block model of microminipig. J. Pharmacol. Sci. 2011;115:122–126. doi: 10.1254/jphs.10R21FM. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

