ARAG, an Antioxidant-Rich Gel, Shows Superiority to Mepilex Ag in the Treatment of Deep Partial Thickness Burns without Sacrificing Antimicrobial Efficiency
- PMID: 37371906
- PMCID: PMC10295424
- DOI: 10.3390/antiox12061176
ARAG, an Antioxidant-Rich Gel, Shows Superiority to Mepilex Ag in the Treatment of Deep Partial Thickness Burns without Sacrificing Antimicrobial Efficiency
Abstract
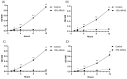
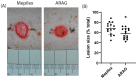
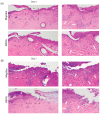
Current treatments for deep tissue burns are limited, and most serve only to enhance hydration or prevent bacterial growth. This leaves burn healing dependent on slow natural processes to debride the wound and reestablish the epidermal and dermal layers of the skin. Infections are well known to destabilize this process through a variety of mechanisms, most notably through increased inflammation and the resulting oxidative stress. In this study, we show that ARAG (an antioxidant-rich antimicrobial gel) can suppress the growth of multiple bacteria commonly found to infect burns (Klebsiella pneumoniae, Proteus vulgaris, Pseudomonas aeruginosa, and Staphylococcus aureus). This inhibition is comparable to that conferred by silver ion release from burn dressings such as Mepilex-Ag. We further show, using a porcine model for deep partial-thickness burns, that ARAG allows for enhanced wound healing over Mepilex-Ag, the current standard of care. Histological findings indicate this is likely due to increased wound debridement and dampening of late inflammatory processes, leading to more balanced physiologic healing. Taken together, these findings show promise for ARAG as a superior alternative to the current standard of care.
Keywords: TPGS; antimicrobial; antioxidant; burns; deep partial thickness burns; wound healing.
Conflict of interest statement
William A. Clark is a stakeholder in AOXbio and the holder of the patent for Lavengel, a product derived from ARAG. All other researchers declare no conflict of interest.
Figures

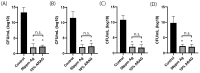

Similar articles
-
An open, parallel, randomized, comparative, multicenter investigation evaluating the efficacy and tolerability of Mepilex Ag versus silver sulfadiazine in the treatment of deep partial-thickness burn injuries.J Trauma Acute Care Surg. 2015 May;78(5):1000-7. doi: 10.1097/TA.0000000000000620. J Trauma Acute Care Surg. 2015. PMID: 25909422 Clinical Trial.
-
The Optimal Treatment for Partial Thickness Burns: A Cost-Utility Analysis of Skin Allograft vs. Topical Silver Dressings.J Burn Care Res. 2020 May 2;41(3):450-456. doi: 10.1093/jbcr/iraa003. J Burn Care Res. 2020. PMID: 32043154
-
Nano-silver modified porcine small intestinal submucosa for the treatment of infected partial-thickness burn wounds.Burns. 2019 Jun;45(4):950-956. doi: 10.1016/j.burns.2018.12.002. Epub 2018 Dec 27. Burns. 2019. PMID: 30595540
-
A retrospective review of burn dressings on a porcine burn model.Burns. 2010 Aug;36(5):680-7. doi: 10.1016/j.burns.2009.06.200. Epub 2009 Oct 27. Burns. 2010. PMID: 19864074 Review.
-
Dressings for superficial and partial thickness burns.Cochrane Database Syst Rev. 2008 Oct 8;(4):CD002106. doi: 10.1002/14651858.CD002106.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2013 Mar 28;(3):CD002106. doi: 10.1002/14651858.CD002106.pub4. PMID: 18843629 Updated. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

