Insights into energy balance dysregulation from a mouse model of methylmalonic aciduria
- PMID: 37369025
- PMCID: PMC10460489
- DOI: 10.1093/hmg/ddad100
Insights into energy balance dysregulation from a mouse model of methylmalonic aciduria
Abstract
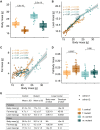
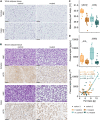
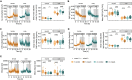
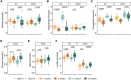
Inherited disorders of mitochondrial metabolism, including isolated methylmalonic aciduria, present unique challenges to energetic homeostasis by disrupting energy-producing pathways. To better understand global responses to energy shortage, we investigated a hemizygous mouse model of methylmalonyl-CoA mutase (Mmut)-type methylmalonic aciduria. We found Mmut mutant mice to have reduced appetite, energy expenditure and body mass compared with littermate controls, along with a relative reduction in lean mass but increase in fat mass. Brown adipose tissue showed a process of whitening, in line with lower body surface temperature and lesser ability to cope with cold challenge. Mutant mice had dysregulated plasma glucose, delayed glucose clearance and a lesser ability to regulate energy sources when switching from the fed to fasted state, while liver investigations indicated metabolite accumulation and altered expression of peroxisome proliferator-activated receptor and Fgf21-controlled pathways. Together, these shed light on the mechanisms and adaptations behind energy imbalance in methylmalonic aciduria and provide insight into metabolic responses to chronic energy shortage, which may have important implications for disease understanding and patient management.
© The Author(s) 2023. Published by Oxford University Press.
Figures


Similar articles
-
Tricarboxylic acid cycle enzyme activities in a mouse model of methylmalonic aciduria.Mol Genet Metab. 2019 Dec;128(4):444-451. doi: 10.1016/j.ymgme.2019.10.007. Epub 2019 Oct 17. Mol Genet Metab. 2019. PMID: 31648943 Free PMC article.
-
In-depth phenotyping reveals common and novel disease symptoms in a hemizygous knock-in mouse model (Mut-ko/ki) of mut-type methylmalonic aciduria.Biochim Biophys Acta Mol Basis Dis. 2020 Mar 1;1866(3):165622. doi: 10.1016/j.bbadis.2019.165622. Epub 2019 Nov 23. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31770620
-
Cellular and computational models reveal environmental and metabolic interactions in MMUT-type methylmalonic aciduria.J Inherit Metab Dis. 2023 May;46(3):421-435. doi: 10.1002/jimd.12575. Epub 2022 Nov 23. J Inherit Metab Dis. 2023. PMID: 36371683
-
Treatment of metabolic disorders using genomic technologies: Lessons from methylmalonic acidemia.J Inherit Metab Dis. 2022 Sep;45(5):872-888. doi: 10.1002/jimd.12534. Epub 2022 Jul 21. J Inherit Metab Dis. 2022. PMID: 35766386 Review.
-
Recent advances in the inherited methylmalonic acidemias.Acta Paediatr Scand. 1987 Sep;76(5):689-96. doi: 10.1111/j.1651-2227.1987.tb10551.x. Acta Paediatr Scand. 1987. PMID: 2889315 Review.
Cited by
-
Vitamin B12 status and skeletal muscle function among elderly: A literature review and pilot study on the effect of oral vitamin B12 supplementation in improving muscle function.Aging Med (Milton). 2024 Aug 17;7(4):480-489. doi: 10.1002/agm2.12346. eCollection 2024 Aug. Aging Med (Milton). 2024. PMID: 39234201 Free PMC article.
-
Lipodystrophy in methylmalonic acidemia associated with elevated FGF21 and abnormal methylmalonylation.JCI Insight. 2024 Feb 22;9(4):e174097. doi: 10.1172/jci.insight.174097. JCI Insight. 2024. PMID: 38271099 Free PMC article.
References
-
- Kölker, S., Schwab, M., Hörster, F., Sauer, S., Hinz, A., Wolf, N.I., Mayatepek, E., Hoffmann, G.F., Smeitink, J.A.M. and Okun, J.G. (2003) Methylmalonic acid, a biochemical Hallmark of Methylmalonic acidurias but no inhibitor of mitochondrial respiratory chain. J. Biol. Chem., 278, 47388–47393. - PubMed
-
- Schwab, M.A., Sauer, S.W., Okun, J.G., Nijtmans, L.G.J., Rodenburg, R.J.T., van den Heuvel, L.P., Dröse, S., Brandt, U., Hoffmann, G.F., Ter Laak, H. et al. (2006) Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins. Biochem. J., 398, 107–112. - PMC - PubMed
-
- Okun, J.G., Hörster, F., Farkas, L.M., Feyh, P., Hinz, A., Sauer, S., Hoffmann, G.F., Unsicker, K., Mayatepek, E. and Kölker, S. (2002) Neurodegeneration in Methylmalonic aciduria involves inhibition of complex II and the tricarboxylic acid cycle, and synergistically acting Excitotoxicity. J. Biol. Chem., 277, 14674–14680. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

