Preservation of developmental spontaneous activity enables early auditory system maturation in deaf mice
- PMID: 37368868
- PMCID: PMC10298803
- DOI: 10.1371/journal.pbio.3002160
Preservation of developmental spontaneous activity enables early auditory system maturation in deaf mice
Abstract
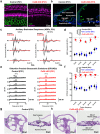
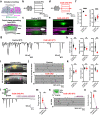
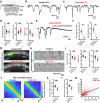
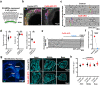
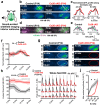
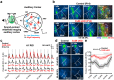
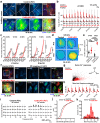
Intrinsically generated neural activity propagates through the developing auditory system to promote maturation and refinement of sound processing circuits prior to hearing onset. This early patterned activity is induced by non-sensory supporting cells in the organ of Corti, which are highly interconnected through gap junctions containing connexin 26 (Gjb2). Although loss of function mutations in Gjb2 impair cochlear development and are the most common cause of congenital deafness, it is not known if these variants disrupt spontaneous activity and the developmental trajectory of sound processing circuits in the brain. Here, we show in a new mouse model of Gjb2-mediated congenital deafness that cochlear supporting cells adjacent to inner hair cells (IHCs) unexpectedly retain intercellular coupling and the capacity to generate spontaneous activity, exhibiting only modest deficits prior to hearing onset. Supporting cells lacking Gjb2 elicited coordinated activation of IHCs, leading to coincident bursts of activity in central auditory neurons that will later process similar frequencies of sound. Despite alterations in the structure of the sensory epithelium, hair cells within the cochlea of Gjb2-deficient mice were intact and central auditory neurons could be activated within appropriate tonotopic domains by loud sounds at hearing onset, indicating that early maturation and refinement of auditory circuits was preserved. Only after cessation of spontaneous activity following hearing onset did progressive hair cell degeneration and enhanced auditory neuron excitability manifest. This preservation of cochlear spontaneous neural activity in the absence of connexin 26 may increase the effectiveness of early therapeutic interventions to restore hearing.
Copyright: © 2023 Kersbergen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: DEB is a paid consultant of Decibel Therapeutics.
Figures

Similar articles
-
Priming central sound processing circuits through induction of spontaneous activity in the cochlea before hearing onset.Trends Neurosci. 2024 Jul;47(7):522-537. doi: 10.1016/j.tins.2024.04.007. Epub 2024 May 22. Trends Neurosci. 2024. PMID: 38782701 Review.
-
Purinergic Signaling Controls Spontaneous Activity in the Auditory System throughout Early Development.J Neurosci. 2021 Jan 27;41(4):594-612. doi: 10.1523/JNEUROSCI.2178-20.2020. Epub 2020 Dec 10. J Neurosci. 2021. PMID: 33303678 Free PMC article.
-
Purinergic signaling in cochlear supporting cells reduces hair cell excitability by increasing the extracellular space.Elife. 2020 Jan 8;9:e52160. doi: 10.7554/eLife.52160. Elife. 2020. PMID: 31913121 Free PMC article.
-
Timed conditional null of connexin26 in mice reveals temporary requirements of connexin26 in key cochlear developmental events before the onset of hearing.Neurobiol Dis. 2015 Jan;73:418-27. doi: 10.1016/j.nbd.2014.09.005. Epub 2014 Sep 22. Neurobiol Dis. 2015. PMID: 25251605
-
Spontaneous activity in the developing auditory system.Cell Tissue Res. 2015 Jul;361(1):65-75. doi: 10.1007/s00441-014-2007-5. Epub 2014 Oct 9. Cell Tissue Res. 2015. PMID: 25296716 Free PMC article. Review.
Cited by
-
Priming central sound processing circuits through induction of spontaneous activity in the cochlea before hearing onset.Trends Neurosci. 2024 Jul;47(7):522-537. doi: 10.1016/j.tins.2024.04.007. Epub 2024 May 22. Trends Neurosci. 2024. PMID: 38782701 Review.
-
Mime: A flexible machine-learning framework to construct and visualize models for clinical characteristics prediction and feature selection.Comput Struct Biotechnol J. 2024 Jun 29;23:2798-2810. doi: 10.1016/j.csbj.2024.06.035. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39055398 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

