Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores
- PMID: 37367618
- PMCID: PMC10305343
- DOI: 10.3390/jof9060682
Characterization of Lung Inflammatory Response to Aspergillus fumigatus Spores
Abstract
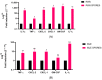
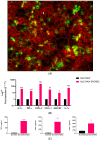
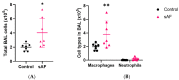
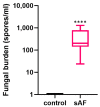
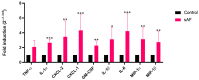
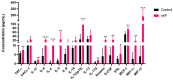
The airway exposure to Aspergillus fumigatus spores (AFsp) is associated with an inflammatory response, potentially leading to allergic and/or chronic pulmonary aspergillosis. The aim of our study is to better understand the host response, first in vitro, then in vivo, following the chronic exposure of mice to AFsp. We investigated the inflammatory response to AFsp in cell mono- and co-culture systems with murine macrophages and alveolar epithelial cells. The mice were subjected to two intranasal instillations using 105 AFsp. Their lungs were processed for inflammatory and histopathological analyses. In cell culture, the gene expressions significantly increased for TNF-α, CXCL-1, CXCL-2, IL-1β, IL-1α and GM-CSF in macrophages, with these increases being limited for TNF-α, CXCL-1 and IL-1α in epithelial cells. In co-culture, increases in the TNF-α, CXCL-2 and CXCL-1 gene expressions were observed to be associated with increased protein levels. The in vivo lung histological analyses of mice challenged by AFsp showed cellular infiltrates in the peribronchial and/or alveolar spaces. A Bio-Plex approach on the bronchoalveolar lavage revealed significant increases in the protein secretion of selected mediators of the challenged mice compared to the unchallenged mice. In conclusion, the exposure to AFsp resulted in a marked inflammatory response of macrophages and epithelial cells. These inflammatory findings were confirmed in mouse models associated with lung histologic changes.
Keywords: Aspergillus fumigatus; epithelial cell; immune response; lung; macrophages; spores.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Vitamin D reduces autophagy by regulating NF-κB resistance to Aspergillus fumigatus infection.Gene. 2020 Aug 30;753:144819. doi: 10.1016/j.gene.2020.144819. Epub 2020 May 30. Gene. 2020. PMID: 32485309
-
Chlorine gas exposure increases susceptibility to invasive lung fungal infection.Am J Physiol Lung Cell Mol Physiol. 2013 Jun 1;304(11):L765-73. doi: 10.1152/ajplung.00030.2013. Epub 2013 Apr 5. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23564508 Free PMC article.
-
Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment.Med Mycol. 1999 Jun;37(3):183-94. doi: 10.1046/j.1365-280x.1999.00219.x. Med Mycol. 1999. PMID: 10421850
-
Three-Dimensional Light Sheet Fluorescence Microscopy of Lungs To Dissect Local Host Immune-Aspergillus fumigatus Interactions.mBio. 2020 Feb 4;11(1):e02752-19. doi: 10.1128/mBio.02752-19. mBio. 2020. PMID: 32019790 Free PMC article.
-
PU.1-CD23 signaling mediates pulmonary innate immunity against Aspergillus fumigatus infection by driving inflammatory response.BMC Immunol. 2023 Jan 17;24(1):4. doi: 10.1186/s12865-023-00539-2. BMC Immunol. 2023. PMID: 36650424 Free PMC article.
Cited by
-
Nanotechnology-Based Drug Delivery Systems to Control Bacterial-Biofilm-Associated Lung Infections.Pharmaceutics. 2023 Nov 3;15(11):2582. doi: 10.3390/pharmaceutics15112582. Pharmaceutics. 2023. PMID: 38004561 Free PMC article. Review.
References
-
- Lauruschkat C.D., Etter S., Schnack E., Ebel F., Schäuble S., Page L., Rümens D., Dragan M., Schlegel N., Panagiotou G., et al. Chronic Occupational Mold Exposure Drives Expansion of Aspergillus-Reactive Type 1 and Type 2 T-Helper Cell Responses. J. Fungi. 2021;7:698. doi: 10.3390/jof7090698. - DOI - PMC - PubMed
-
- Camara B., Reymond E., Saint-Raymond C., Roth H., Brenier-Pinchart M.-P., Pinel C., Cadranel J., Ferretti G., Pelloux H., Pison C., et al. Characteristics and outcomes of chronic pulmonary aspergillosis: A retrospective analysis of a tertiary hospital registry: Chronic pulmonary aspergillosis in non-immunocompromised patients. Clin. Respir. J. 2015;9:65–73. doi: 10.1111/crj.12105. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

