Ocular-Surface Regeneration Therapies for Eye Disorders: The State of the Art
- PMID: 37366796
- PMCID: PMC10295950
- DOI: 10.3390/biotech12020048
Ocular-Surface Regeneration Therapies for Eye Disorders: The State of the Art
Abstract
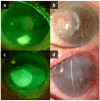
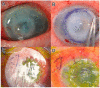
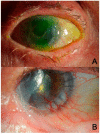
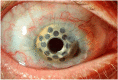
The ocular surface is a complex structure that includes cornea, conjunctiva, limbus, and tear film, and is critical for maintaining visual function. When the ocular-surface integrity is altered by a disease, conventional therapies usually rely on topical drops or tissue replacement with more invasive procedures, such as corneal transplants. However, in the last years, regeneration therapies have emerged as a promising approach to repair the damaged ocular surface by stimulating cell proliferation and restoring the eye homeostasis and function. This article reviews the different strategies employed in ocular-surface regeneration, including cell-based therapies, growth-factor-based therapies, and tissue-engineering approaches. Dry eye and neurotrophic keratopathy diseases can be treated with nerve-growth factors to stimulate the limbal stem-cell proliferation and the corneal nerve regeneration, whereas conjunctival autograft or amniotic membrane are used in subjects with corneal limbus dysfunction, such as limbal stem-cell deficiency or pterygium. Further, new therapies are available for patients with corneal endothelium diseases to promote the expansion and migration of cells without the need of corneal keratoplasty. Finally, gene therapy is a promising new frontier of regeneration medicine that can modify the gene expression and, potentially, restore the corneal transparency by reducing fibrosis and neovascularization, as well as by stimulating stem-cell proliferation and tissue regeneration.
Keywords: amniotic membrane; autologous serum tear; dry-eye disease; limbal stem cells; neurotrophic keratopathy; ocular-surface disease; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Limbal stem cell transplantation: an evidence-based analysis.Ont Health Technol Assess Ser. 2008;8(7):1-58. Epub 2008 Oct 1. Ont Health Technol Assess Ser. 2008. PMID: 23074512 Free PMC article.
-
[Amniotic membrane graft in ocular surface disease. Prospective study with 31 cases].J Fr Ophtalmol. 2001 Oct;24(8):798-812. J Fr Ophtalmol. 2001. PMID: 11894530 French.
-
Cultured corneal epithelia for ocular surface disease.Trans Am Ophthalmol Soc. 1999;97:891-986. Trans Am Ophthalmol Soc. 1999. PMID: 10703147 Free PMC article.
-
Surgery of the cornea: corneal, limbal stem cell and amniotic membrane transplantation.Dev Ophthalmol. 2008;41:159-170. doi: 10.1159/000131087. Dev Ophthalmol. 2008. PMID: 18453767 Review.
-
Science and Art of Cell-Based Ocular Surface Regeneration.Int Rev Cell Mol Biol. 2015;319:45-106. doi: 10.1016/bs.ircmb.2015.07.001. Int Rev Cell Mol Biol. 2015. PMID: 26404466 Review.
Cited by
-
Characterization of Central and Nasal Orbital Adipose Stem Cells and their Neural Differentiation Footprints.Curr Stem Cell Res Ther. 2024;19(8):1111-1119. doi: 10.2174/1574888X19666230905114246. Curr Stem Cell Res Ther. 2024. PMID: 37670706
-
Pharmacological Stimulation of Soluble Guanylate Cyclase Counteracts the Profibrotic Activation of Human Conjunctival Fibroblasts.Cells. 2024 Feb 18;13(4):360. doi: 10.3390/cells13040360. Cells. 2024. PMID: 38391973 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
