Exploring the mechanism of JiGuCao capsule formula on treating hepatitis B virus infection via network pharmacology analysis and in vivo/vitro experiment verification
- PMID: 37361218
- PMCID: PMC10285482
- DOI: 10.3389/fphar.2023.1159094
Exploring the mechanism of JiGuCao capsule formula on treating hepatitis B virus infection via network pharmacology analysis and in vivo/vitro experiment verification
Abstract
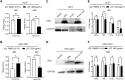
The JiGuCao capsule formula (JCF) has demonstrated promising curative effects in treating chronic hepatitis B (CHB) in clinical trials. Here, we aimed to investigate JCF's function and mechanism in diseases related to the hepatitis B virus (HBV). We used mass spectrometry (MS) to identify the active metabolites of JCF and established the HBV replication mouse model by hydrodynamically injecting HBV replication plasmids into the mice's tail vein. Liposomes were used to transfect the plasmids into the cells. The CCK-8 kit identified cell viability. We detected the levels of HBV s antigen (HBsAg) and HBV e antigen (HBeAg) by the quantitative determination kits. qRT-PCR and Western blot were used to detect the genes' expression. The key pathways and key genes related to JCF on CHB treatment were obtained by network pharmacological analysis. Our results showed that JCF accelerated the elimination of HBsAg in mice. JCF and its medicated serum inhibited HBV replication and proliferation of HBV-replicating hepatoma cells in vitro. And the key targets of JCF in treating CHB were CASP3, CXCL8, EGFR, HSPA8, IL6, MDM2, MMP9, NR3C1, PTGS2, and VEGFA. Furthermore, these key targets were related to pathways in cancer, hepatitis B, microRNAs in cancer, PI3K-Akt signaling, and proteoglycans in cancer pathways. Finally, Cholic Acid, Deoxycholic Acid, and 3', 4', 7-Trihydroxyflavone were the main active metabolites of JCF that we obtained. JCF employed its active metabolites to perform an anti-HBV effect and prevent the development of HBV-related diseases.
Keywords: JiGuCao capsule formula; UHPLC-MS/MS; experimental verification; hepatitis B virus; network pharmacology.
Copyright © 2023 Cao, Zhang, Chen, Wang, Liang, Zhang, Liu, Li, Yao, Jin, Guo, Chen, Gong, Li, Zao and Ye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Anti-hepatitis B virus activity of lithospermic acid, a polyphenol from Salvia miltiorrhiza, in vitro and in vivo by autophagy regulation.J Ethnopharmacol. 2023 Feb 10;302(Pt A):115896. doi: 10.1016/j.jep.2022.115896. Epub 2022 Nov 2. J Ethnopharmacol. 2023. PMID: 36334815
-
Network pharmacology and in vitro experiments-based strategy to investigate the mechanisms of KangXianYiAi formula for hepatitis B virus-related hepatocellular carcinoma.Front Pharmacol. 2022 Sep 5;13:985084. doi: 10.3389/fphar.2022.985084. eCollection 2022. Front Pharmacol. 2022. PMID: 36133813 Free PMC article.
-
The mechanism of TiaoGanYiPi formula for treating chronic hepatitis B by network pharmacology and molecular docking verification.Sci Rep. 2021 Apr 16;11(1):8402. doi: 10.1038/s41598-021-87812-9. Sci Rep. 2021. PMID: 33863948 Free PMC article.
-
Treatment of chronic hepatitis B: case selection and duration of therapy.J Gastroenterol Hepatol. 2002 Apr;17(4):409-14. doi: 10.1046/j.1440-1746.2002.02767.x. J Gastroenterol Hepatol. 2002. PMID: 11982721 Review.
-
Progression and status of antiviral monitoring in patients with chronic hepatitis B: From HBsAg to HBV RNA.World J Hepatol. 2018 Sep 27;10(9):603-611. doi: 10.4254/wjh.v10.i9.603. World J Hepatol. 2018. PMID: 30310538 Free PMC article. Review.
Cited by
-
Application of network pharmacology in traditional Chinese medicine for the treatment of digestive system diseases.Front Pharmacol. 2024 Jul 17;15:1412997. doi: 10.3389/fphar.2024.1412997. eCollection 2024. Front Pharmacol. 2024. PMID: 39086391 Free PMC article. Review.
-
Application of network pharmacology in synergistic action of Chinese herbal compounds.Theory Biosci. 2024 Sep;143(3):195-203. doi: 10.1007/s12064-024-00419-2. Epub 2024 Jun 18. Theory Biosci. 2024. PMID: 38888845 Review.
References
-
- Cao G. Y., Geng S. X., Luo Y., Tian S., Ning B., Zhuang X. S., et al. (2021a). The rapid identification of chemical constituents in Fufang Xiling Jiedu capsule, a modern Chinese medicine, by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight tandem mass spectrometry and data mining strategy. J. Sep. Sci. 44, 1815–1823. 10.1002/jssc.202001093 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

