Synthesis of a Novel Boronic Acid Transition State Inhibitor, MB076: A Heterocyclic Triazole Effectively Inhibits Acinetobacter-Derived Cephalosporinase Variants with an Expanded-Substrate Spectrum
- PMID: 37358467
- PMCID: PMC10350917
- DOI: 10.1021/acs.jmedchem.3c00144
Synthesis of a Novel Boronic Acid Transition State Inhibitor, MB076: A Heterocyclic Triazole Effectively Inhibits Acinetobacter-Derived Cephalosporinase Variants with an Expanded-Substrate Spectrum
Abstract
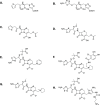
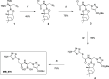
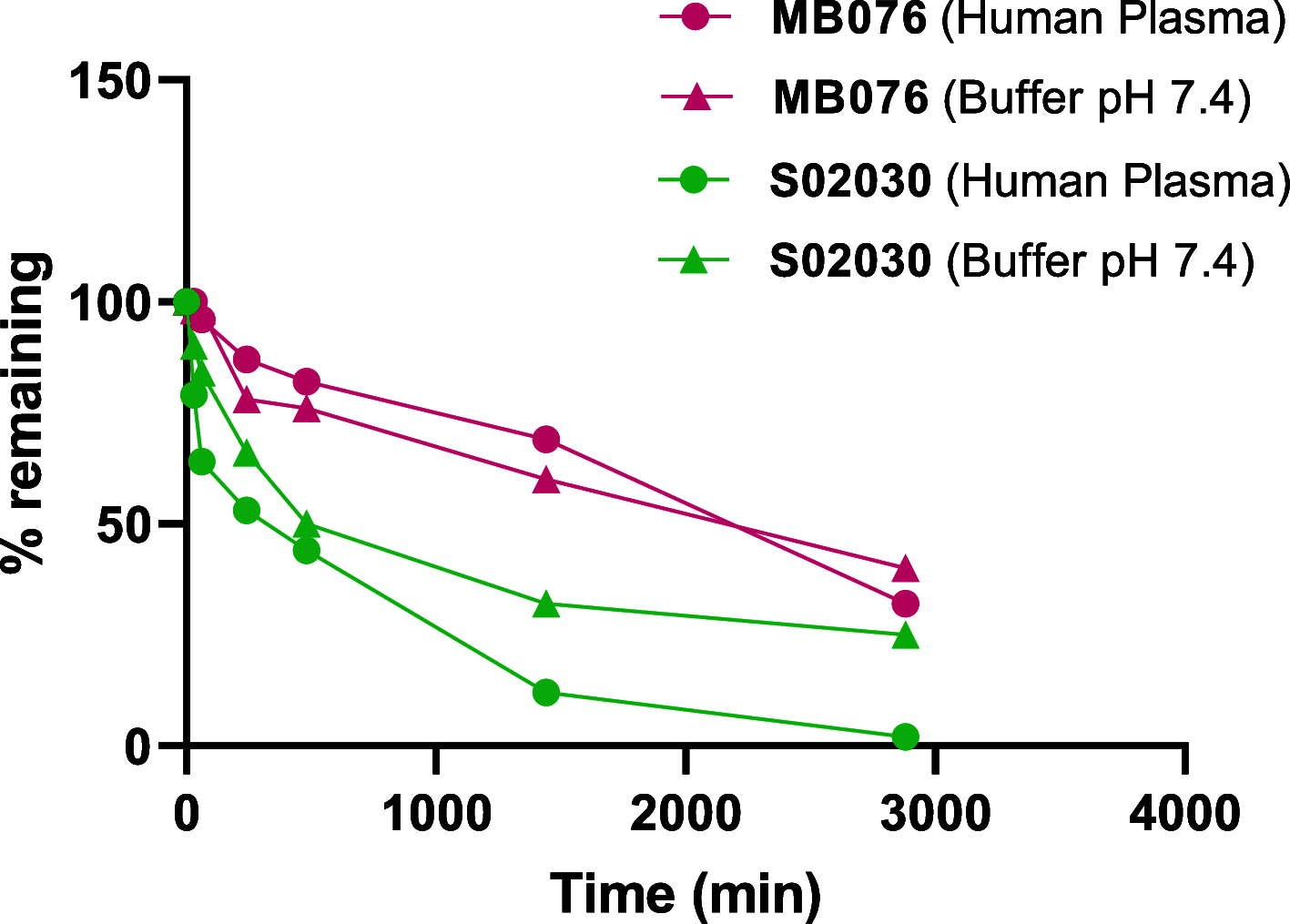
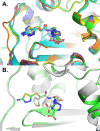
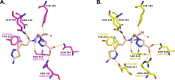
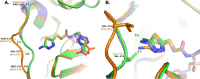
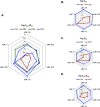
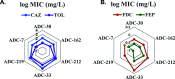
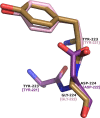
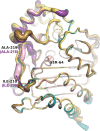
Class C Acinetobacter-derived cephalosporinases (ADCs) represent an important target for inhibition in the multidrug-resistant pathogen Acinetobacter baumannii. Many ADC variants have emerged, and characterization of their structural and functional differences is essential. Equally as important is the development of compounds that inhibit all prevalent ADCs despite these differences. The boronic acid transition state inhibitor, MB076, a novel heterocyclic triazole with improved plasma stability, was synthesized and inhibits seven different ADC β-lactamase variants with Ki values <1 μM. MB076 acted synergistically in combination with multiple cephalosporins to restore susceptibility. ADC variants containing an alanine duplication in the Ω-loop, specifically ADC-33, exhibited increased activity for larger cephalosporins, such as ceftazidime, cefiderocol, and ceftolozane. X-ray crystal structures of ADC variants in this study provide a structural context for substrate profile differences and show that the inhibitor adopts a similar conformation in all ADC variants, despite small changes near their active sites.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
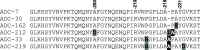
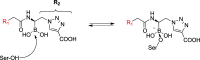
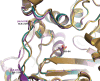
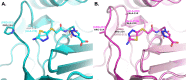
Similar articles
-
Structural Insights into Inhibition of the Acinetobacter-Derived Cephalosporinase ADC-7 by Ceftazidime and Its Boronic Acid Transition State Analog.Antimicrob Agents Chemother. 2020 Nov 17;64(12):e01183-20. doi: 10.1128/AAC.01183-20. Print 2020 Nov 17. Antimicrob Agents Chemother. 2020. PMID: 32988830 Free PMC article.
-
Structure-Based Analysis of Boronic Acids as Inhibitors of Acinetobacter-Derived Cephalosporinase-7, a Unique Class C β-Lactamase.ACS Infect Dis. 2018 Mar 9;4(3):325-336. doi: 10.1021/acsinfecdis.7b00152. Epub 2017 Dec 8. ACS Infect Dis. 2018. PMID: 29144724 Free PMC article.
-
Biochemical and structural analysis of inhibitors targeting the ADC-7 cephalosporinase of Acinetobacter baumannii.Biochemistry. 2014 Dec 9;53(48):7670-9. doi: 10.1021/bi500887n. Epub 2014 Nov 25. Biochemistry. 2014. PMID: 25380506 Free PMC article.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
Prospective role of cefiderocol in the management of carbapenem-resistant Acinetobacter baumannii infections: Review of the evidence.Int J Antimicrob Agents. 2023 Aug;62(2):106882. doi: 10.1016/j.ijantimicag.2023.106882. Epub 2023 Jun 8. Int J Antimicrob Agents. 2023. PMID: 37301312 Review.
Cited by
-
Ethynyl-substituted benzosiloxaboroles: the role of C(π)⋯B interactions in their crystal packing and use in Cu(i)-catalyzed 1,3-dipolar cycloaddition.RSC Adv. 2024 May 17;14(23):16069-16082. doi: 10.1039/d4ra02137a. eCollection 2024 May 15. RSC Adv. 2024. PMID: 38765480 Free PMC article.
-
Prevention and potential remedies for antibiotic resistance: current research and future prospects.Front Microbiol. 2024 Oct 3;15:1455759. doi: 10.3389/fmicb.2024.1455759. eCollection 2024. Front Microbiol. 2024. PMID: 39421555 Free PMC article. Review.
References
-
- Centers for Disease Control . US Department of Health and Human Services, COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; Atlanta, GA, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

