Long non‑coding RNAs as potential therapeutic targets in non‑small cell lung cancer (Review)
- PMID: 37350412
- PMCID: PMC10413047
- DOI: 10.3892/ijmm.2023.5271
Long non‑coding RNAs as potential therapeutic targets in non‑small cell lung cancer (Review)
Abstract
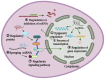
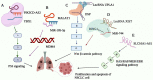
Non‑small cell lung cancer (NSCLC) is one of the most common malignancies with a high morbidity and mortality rate. Long non‑coding RNAs (lncRNAs) have been reported to be closely associated with the occurrence and progression of NSCLC. In addition, lncRNAs have been documented to participate in the development of drug resistance and radiation sensitivity in patients with NSCLC. Due to their extensive functional characterization, high tissue specificity and sex specificity, lncRNAs have been proposed to be novel biomarkers and therapeutic targets for NSCLC. Therefore, in the current review, the functional classification of lncRNAs were presented, whilst the potential roles of lncRNAs in NSCLC were also summarized. Various physiological aspects, including proliferation, invasion and drug resistance, were all discussed. It is anticipated that the present review will provide a perspective on lncRNAs as potential diagnostic molecular biomarkers and therapeutic targets for NSCLC.
Keywords: NSCLC; biomarkers; drug resistance; lncRNA; therapeutic targets.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Long noncoding RNAs: new insights into non-small cell lung cancer biology, diagnosis and therapy.Med Oncol. 2016 Feb;33(2):18. doi: 10.1007/s12032-016-0731-2. Epub 2016 Jan 19. Med Oncol. 2016. PMID: 26786153 Review.
-
Long Noncoding RNA LINC00460 Promotes the Gefitinib Resistance of Nonsmall Cell Lung Cancer Through Epidermal Growth Factor Receptor by Sponging miR-769-5p.DNA Cell Biol. 2019 Feb;38(2):176-183. doi: 10.1089/dna.2018.4462. Epub 2019 Jan 2. DNA Cell Biol. 2019. PMID: 30601026 Free PMC article.
-
Construction of a competing endogenous RNA network using differentially expressed lncRNAs, miRNAs and mRNAs in non‑small cell lung cancer.Oncol Rep. 2019 Dec;42(6):2402-2415. doi: 10.3892/or.2019.7378. Epub 2019 Oct 17. Oncol Rep. 2019. PMID: 31638248 Free PMC article.
-
New insights into long non-coding RNAs in non-small cell lung cancer.Biomed Pharmacother. 2020 Nov;131:110775. doi: 10.1016/j.biopha.2020.110775. Epub 2020 Sep 26. Biomed Pharmacother. 2020. PMID: 33152934 Review.
-
Long non‑coding RNA TP73‑AS1 accelerates the progression and cisplatin resistance of non‑small cell lung cancer by upregulating the expression of TRIM29 via competitively targeting microRNA‑34a‑5p.Mol Med Rep. 2020 Nov;22(5):3822-3832. doi: 10.3892/mmr.2020.11473. Epub 2020 Sep 1. Mol Med Rep. 2020. PMID: 32901838 Free PMC article.
Cited by
-
Current Views on Pathophysiology and Potential Therapeutic Targets in Sjögren's Syndrome: A Review from the Perspective of Viral Infections, Toll-like Receptors, and Long-Noncoding RNAs.J Clin Med. 2023 Sep 10;12(18):5873. doi: 10.3390/jcm12185873. J Clin Med. 2023. PMID: 37762814 Free PMC article. Review.
-
Non-coding RNAs and exosomal non-coding RNAs in lung cancer: insights into their functions.Front Cell Dev Biol. 2024 May 27;12:1397788. doi: 10.3389/fcell.2024.1397788. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38859962 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

