Comparison of the therapeutic effects between stem cells and exosomes in primary ovarian insufficiency: as promising as cells but different persistency and dosage
- PMID: 37340468
- PMCID: PMC10283237
- DOI: 10.1186/s13287-023-03397-2
Comparison of the therapeutic effects between stem cells and exosomes in primary ovarian insufficiency: as promising as cells but different persistency and dosage
Abstract
Background: Primary ovarian insufficiency (POI) refers to the loss of ovarian function under the age of 40 and results in amenorrhea and infertility. Our previous studies have shown that transplantation of mesenchymal stem cells (MSCs) and MSC-derived exosomes in chemotherapy-induced POI mouse ovaries can reverse the POI and eventually achieve pregnancy. Based on our recent studies, MSC-derived exosomes have almost equal therapeutic potentials as transplanted MSCs. However, it is still unclear whether exosomes can completely replace MSCs in POI treatment. For the reliable application of cell-free treatment for POI patients using exosomes, there is a need to understand whether there is any outcome and effectiveness difference between MSC and MSC-derived exosome treatment.
Methods: Comparing the therapeutic effect of intravenous injection using MSCs and equal amounts of exosomes in a POI mouse model will reveal the difference between the two therapeutic resources. In this study, we induced POI in C57/BL6 mice by chemotherapy (CXT) using a standard protocol. We then injected four different doses of MSCs or equal amounts of commercialized MSC-derived exosomes by retro-orbital injection post-CXT.
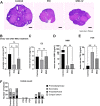
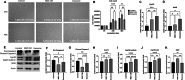
Result: After MSC/exosome treatment, tissue and serum samples were harvested to analyze molecular changes after treatment, while other mice in parallel experiments underwent breeding experiments to compare the restoration of fertility. Both the MSC- and exosome-treated groups had a restored estrous cycle and serum hormone levels compared to untreated POI mice. The pregnancy rate in the MSC-treated group was 60-100% after treatment, while the pregnancy rate in the exosome-treated group was 30-50% after treatment. Interestingly, in terms of long-term effects, MSC-treated mice still showed a 60-80% pregnancy rate in the second round of breeding, while the exosome-treated group became infertile again in the second round of breeding.
Conclusions: Although there were some differences in the efficacy between MSC treatment and exosome treatment, both treatments were able to achieve pregnancy in the POI mouse model. In conclusion, we report that MSC-derived exosomes are a promising therapeutic option to restore ovarian function in POI conditions similar to treatment with MSCs.
Keywords: Exosome; Infertility; Long-term effect; Mesenchymal stem cell; Primary ovarian insufficiency.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism.Stem Cell Res Ther. 2019 Jan 25;10(1):46. doi: 10.1186/s13287-019-1136-x. Stem Cell Res Ther. 2019. PMID: 30683144 Free PMC article.
-
Safety of Intraovarian Injection of Human Mesenchymal Stem Cells in a Premature Ovarian Insufficiency Mouse Model.Cell Transplant. 2021 Jan-Dec;30:963689720988502. doi: 10.1177/0963689720988502. Cell Transplant. 2021. PMID: 33593078 Free PMC article.
-
Fertility protection: a novel approach using pretreatment with mesenchymal stem cell exosomes to prevent chemotherapy-induced ovarian damage in a mouse model.Am J Obstet Gynecol. 2024 Jul;231(1):111.e1-111.e18. doi: 10.1016/j.ajog.2024.02.023. Epub 2024 Feb 18. Am J Obstet Gynecol. 2024. PMID: 38378099
-
Menstrual blood-derived endometrial stem cell, a unique and promising alternative in the stem cell-based therapy for chemotherapy-induced premature ovarian insufficiency.Stem Cell Res Ther. 2023 Nov 13;14(1):327. doi: 10.1186/s13287-023-03551-w. Stem Cell Res Ther. 2023. PMID: 37957675 Free PMC article. Review.
-
Human UC-MSC-derived exosomes facilitate ovarian renovation in rats with chemotherapy-induced premature ovarian insufficiency.Front Endocrinol (Lausanne). 2023 Jul 26;14:1205901. doi: 10.3389/fendo.2023.1205901. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37564988 Free PMC article. Review.
Cited by
-
Use of mesenchymal stem cells to enhance or restore fertility potential: a systematic review of available experimental strategies.Hum Reprod Open. 2023 Oct 25;2023(4):hoad040. doi: 10.1093/hropen/hoad040. eCollection 2023. Hum Reprod Open. 2023. PMID: 37954935 Free PMC article. Review.
-
Evaluation of secretomes derived from human dermal and adipose tissue mesenchymal stem/stromal cells for skin wound healing: not as effective as cells.Stem Cell Res Ther. 2024 Jan 17;15(1):15. doi: 10.1186/s13287-023-03630-y. Stem Cell Res Ther. 2024. PMID: 38229157 Free PMC article.
-
Granulosa cell insight: unraveling the potential of menstrual blood-derived stem cells and their exosomes on mitochondrial mechanisms in polycystic ovary syndrome (PCOS).J Ovarian Res. 2024 Aug 17;17(1):167. doi: 10.1186/s13048-024-01484-3. J Ovarian Res. 2024. PMID: 39153978 Free PMC article.
-
Conservative Hypomethylation of Mesenchymal Stem Cells and Their Secretome Restored the Follicular Development in Cisplatin-Induced Premature Ovarian Failure Mice.Reprod Sci. 2024 Apr;31(4):1053-1068. doi: 10.1007/s43032-023-01389-4. Epub 2023 Nov 13. Reprod Sci. 2024. PMID: 37957472 Free PMC article.
-
A Comparative Analysis of Naïve Exosomes and Enhanced Exosomes with a Focus on the Treatment Potential in Ovarian Disorders.J Pers Med. 2024 Apr 30;14(5):482. doi: 10.3390/jpm14050482. J Pers Med. 2024. PMID: 38793064 Free PMC article.
References
-
- Elfayomy AK, Almasry SM, El-Tarhouny SA, Eldomiaty MA. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: possible direct and indirect effects. Tissue Cell. 2016;48(4):370–382. doi: 10.1016/j.tice.2016.05.001. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

