The secret life of chromatin tethers
- PMID: 37339933
- PMCID: PMC10730768
- DOI: 10.1002/1873-3468.14685
The secret life of chromatin tethers
Abstract
The nuclear envelope plays an essential role in organizing the genome inside of the nucleus. The inner nuclear membrane is coated with a meshwork of filamentous lamin proteins that provide a surface to organize a variety of cellular processes. A subset of nuclear lamina- and membrane-associated proteins functions as anchors to hold transcriptionally silent heterochromatin at the nuclear periphery. While most chromatin tethers are integral membrane proteins, a limited number are lamina-bound. One example is the mammalian proline-rich 14 (PRR14) protein. PRR14 is a recently characterized protein with unique function that is different from other known chromatin tethers. Here, we review our current understanding of PRR14 structure and function in organizing heterochromatin at the nuclear periphery.
Keywords: HP1; LADs; PRR14; nuclear lamina; peripheral heterochromatin.
© 2023 Federation of European Biochemical Societies.
Figures

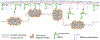
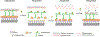
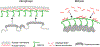
Similar articles
-
The human protein PRR14 tethers heterochromatin to the nuclear lamina during interphase and mitotic exit.Cell Rep. 2013 Oct 31;5(2):292-301. doi: 10.1016/j.celrep.2013.09.024. Cell Rep. 2013. PMID: 24209742 Free PMC article.
-
PRR14 organizes H3K9me3-modified heterochromatin at the nuclear lamina.Nucleus. 2023 Dec;14(1):2165602. doi: 10.1080/19491034.2023.2165602. Nucleus. 2023. PMID: 36633363 Free PMC article.
-
Specifying peripheral heterochromatin during nuclear lamina reassembly.Nucleus. 2014 Jan-Feb;5(1):32-9. doi: 10.4161/nucl.28167. Epub 2014 Feb 10. Nucleus. 2014. PMID: 24637393 Free PMC article. Review.
-
The PRR14 heterochromatin tether encodes modular domains that mediate and regulate nuclear lamina targeting.J Cell Sci. 2020 May 27;133(10):jcs240416. doi: 10.1242/jcs.240416. J Cell Sci. 2020. PMID: 32317397 Free PMC article.
-
Choreography of lamina-associated domains: structure meets dynamics.FEBS Lett. 2023 Nov;597(22):2806-2822. doi: 10.1002/1873-3468.14771. Epub 2023 Nov 14. FEBS Lett. 2023. PMID: 37953467 Free PMC article. Review.
References
-
- Guttinger S, Laurell E, Kutay U, Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol 10, 178–191 (2009). - PubMed
-
- Wong X, Luperchio TR, Reddy KL, NET gains and losses: the role of changing nuclear envelope proteomes in genome regulation. Curr Opin Cell Biol 28, 105–120 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

