Association of epilepsy, anti-epileptic drugs (AEDs), and type 2 diabetes mellitus (T2DM): a population-based cohort retrospective study, impact of AEDs on T2DM-related molecular pathway, and via peroxisome proliferator-activated receptor γ transactivation
- PMID: 37334286
- PMCID: PMC10272786
- DOI: 10.3389/fendo.2023.1156952
Association of epilepsy, anti-epileptic drugs (AEDs), and type 2 diabetes mellitus (T2DM): a population-based cohort retrospective study, impact of AEDs on T2DM-related molecular pathway, and via peroxisome proliferator-activated receptor γ transactivation
Abstract
Introduction: A potential association between epilepsy and subsequent type 2 diabetes mellitus (T2DM) has emerged in recent studies. However, the association between epilepsy, anti-epileptic drugs (AEDs), and the risk of T2DM development remains controversial. We aimed to conduct a nationwide, population-based, retrospective, cohort study to evaluate this relationship.
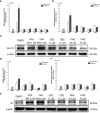
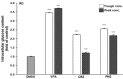
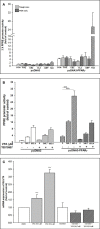
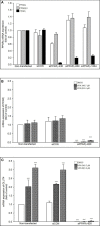
Methods: We extracted data from the Taiwan Longitudinal Generation Tracking Database of patients with new-onset epilepsy and compared it with that of a comparison cohort of patients without epilepsy. A Cox proportional hazards regression model was used to analyze the difference in the risk of developing T2DM between the two cohorts. Next-generation RNA sequencing was used to characterize T2DM-related molecularchanges induced by AEDs and the T2DM-associated pathways they alter. The potential of AEDs to induce peroxisome proliferator-activated receptor γ (PPARγ) transactivation was also evaluated.
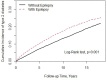
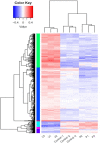
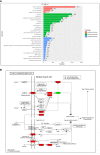
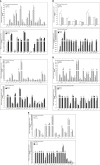
Results: After adjusting for comorbidities and confounding factors, the case group (N = 14,089) had a higher risk for T2DM than the control group (N = 14,089) [adjusted hazards ratio (aHR), 1.27]. Patients with epilepsy not treated with AEDs exhibited a significantly higher risk of T2DM (aHR, 1.70) than non-epileptic controls. In those treated with AEDs, the risk of developing T2DM was significantly lower than in those not treated (all aHR ≤ 0.60). However, an increase in the defined daily dose of phenytoin (PHE), but not of valproate (VPA), increased the risk of T2DM development (aHR, 2.28). Functional enrichment analysis of differentially expressed genes showed that compared to PHE, VPA induced multiple beneficial genes associated with glucose homeostasis. Among AEDs, VPA induced the specific transactivation of PPARγ.
Discussion: Our study shows epilepsy increases the risk of T2DM development, however, some AEDs such as VPA might yield a protective effect against it. Thus, screening blood glucose levels in patients with epilepsy is required to explore the specific role and impact of AEDs in the development of T2DM. Future in depth research on the possibility to repurpose VPA for the treatment of T2DM, will offer valuable insight regarding the relationship between epilepsy and T2DM.
Keywords: anti-epileptic drugs; epilepsy; next-generation RNA sequencing; peroxisome proliferator-activated receptor γ; type 2 diabetes mellitus.
Copyright © 2023 Tien, Wu, Lin, Chu, Wang, Hsu, Tsai, Fang and Lim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
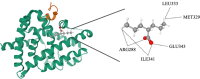
Figures

Similar articles
-
Effects of antiepileptic drugs on lipogenic gene regulation and hyperlipidemia risk in Taiwan: a nationwide population-based cohort study and supporting in vitro studies.Arch Toxicol. 2018 Sep;92(9):2829-2844. doi: 10.1007/s00204-018-2263-3. Epub 2018 Jul 13. Arch Toxicol. 2018. PMID: 30003287
-
Polymorphisms of peroxisome proliferator-activated receptor γ (PPARγ) and cluster of differentiation 36 (CD36) associated with valproate-induced obesity in epileptic patients.Psychopharmacology (Berl). 2018 Sep;235(9):2665-2673. doi: 10.1007/s00213-018-4960-2. Epub 2018 Jul 8. Psychopharmacology (Berl). 2018. PMID: 29984389
-
Association of antipsychotic drugs on type 2 diabetes mellitus risk in patients with schizophrenia: a population-based cohort and in vitro glucose homeostasis-related gene expression study.BMC Psychiatry. 2024 Oct 30;24(1):751. doi: 10.1186/s12888-024-06222-z. BMC Psychiatry. 2024. PMID: 39472855 Free PMC article.
-
Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child.Cochrane Database Syst Rev. 2014 Oct 30;2014(10):CD010236. doi: 10.1002/14651858.CD010236.pub2. Cochrane Database Syst Rev. 2014. PMID: 25354543 Free PMC article. Review.
-
[The decreased level of plasma carnitine in patients with epilepsy].Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117(6):106-110. doi: 10.17116/jnevro201711761106-110. Zh Nevrol Psikhiatr Im S S Korsakova. 2017. PMID: 28745680 Review. Russian.
Cited by
-
Identifying the genetic association between severe autoimmune type 2 diabetes and the risk of focal epilepsy.Front Endocrinol (Lausanne). 2024 Nov 6;15:1396912. doi: 10.3389/fendo.2024.1396912. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39568813 Free PMC article.
-
Exploring the clinical connections between epilepsy and diabetes mellitus: Promising therapeutic strategies utilizing agmatine and metformin.Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):9617-9632. doi: 10.1007/s00210-024-03295-1. Epub 2024 Jul 27. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39066910 Review.

