Identification of a humanized mouse model for functional testing of immune-mediated biomaterial foreign body response
- PMID: 37327334
- PMCID: PMC10275594
- DOI: 10.1126/sciadv.ade9488
Identification of a humanized mouse model for functional testing of immune-mediated biomaterial foreign body response
Abstract
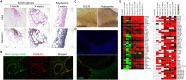
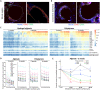
Biomedical devices comprise a major component of modern medicine, however immune-mediated fibrosis and rejection can limit their function over time. Here, we describe a humanized mouse model that recapitulates fibrosis following biomaterial implantation. Cellular and cytokine responses to multiple biomaterials were evaluated across different implant sites. Human innate immune macrophages were verified as essential to biomaterial rejection in this model and were capable of cross-talk with mouse fibroblasts for collagen matrix deposition. Cytokine and cytokine receptor array analysis confirmed core signaling in the fibrotic cascade. Foreign body giant cell formation, often unobserved in mice, was also prominent. Last, high-resolution microscopy coupled with multiplexed antibody capture digital profiling analysis supplied spatial resolution of rejection responses. This model enables the study of human immune cell-mediated fibrosis and interactions with implanted biomaterials and devices.
Figures


Similar articles
-
Foreign body reaction to biomaterials.Semin Immunol. 2008 Apr;20(2):86-100. doi: 10.1016/j.smim.2007.11.004. Epub 2007 Dec 26. Semin Immunol. 2008. PMID: 18162407 Free PMC article. Review.
-
Localized immunosuppressive environment in the foreign body response to implanted biomaterials.Am J Pathol. 2009 Jul;175(1):161-70. doi: 10.2353/ajpath.2009.080962. Epub 2009 Jun 15. Am J Pathol. 2009. PMID: 19528351 Free PMC article.
-
Lipid Deposition Profiles Influence Foreign Body Responses.Adv Mater. 2023 May;35(21):e2205709. doi: 10.1002/adma.202205709. Epub 2023 Mar 31. Adv Mater. 2023. PMID: 36871193 Free PMC article.
-
T lymphocytes as critical mediators in tissue regeneration, fibrosis, and the foreign body response.Acta Biomater. 2021 Oct 1;133:17-33. doi: 10.1016/j.actbio.2021.04.023. Epub 2021 Apr 24. Acta Biomater. 2021. PMID: 33905946 Review.
-
Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates.Nat Mater. 2017 Jun;16(6):671-680. doi: 10.1038/nmat4866. Epub 2017 Mar 20. Nat Mater. 2017. PMID: 28319612 Free PMC article.
Cited by
-
Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead.Nat Rev Endocrinol. 2025 Jan;21(1):14-30. doi: 10.1038/s41574-024-01029-0. Epub 2024 Sep 3. Nat Rev Endocrinol. 2025. PMID: 39227741 Review.
-
Therapeutic Insulin Analogue Concentrations at Infusion Sites Enhanced the Pro-Inflammatory Response and Apoptosis in an In Vitro Macrophage-Material Interaction Model.ACS Pharmacol Transl Sci. 2024 Jul 3;7(8):2544-2556. doi: 10.1021/acsptsci.4c00363. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39156741
-
Immunoengineering Biomaterials for Musculoskeletal Tissue Repair across Lifespan.Adv Mater. 2024 Jul;36(28):e2311646. doi: 10.1002/adma.202311646. Epub 2024 May 7. Adv Mater. 2024. PMID: 38416061 Review.
-
Immunocompatible elastomer with increased resistance to the foreign body response.Nat Commun. 2024 Aug 30;15(1):7526. doi: 10.1038/s41467-024-52023-z. Nat Commun. 2024. PMID: 39214984 Free PMC article.
-
Adhesive anti-fibrotic interfaces on diverse organs.Nature. 2024 Jun;630(8016):360-367. doi: 10.1038/s41586-024-07426-9. Epub 2024 May 22. Nature. 2024. PMID: 38778109 Free PMC article.
References
-
- S. Kurtz, K. Ong, E. Lau, F. Mowat, M. Halpern, Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 89, 780–785 (2007). - PubMed
-
- Institute of Medicine, Board on Population Health and Public Health Practice, Committee on the Public Health Effectiveness of the FDA 510(k) Clearance Process, Medical Devices and the Public's Health: The FDA 510(k) Clearance Process at 35 Years (National Academies Press, 2011), pp. 1–298.
-
- P. de Vos, M. M. Faas, B. Strand, R. Calafiore, Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 27, 5603–5617 (2006). - PubMed
-
- D. Jacobs-Tulleneers-Thevissen, M. Chintinne, Z. Ling, P. Gillard, L. Schoonjans, G. Delvaux, B. L. Strand, F. Gorus, B. Keymeulen, D. Pipeleers; Beta Cell Therapy Consortium EU-FP7 , Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 56, 1605–1614 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

