Uptake and Outcomes of Neoadjuvant Chemotherapy Among US Patients With Less Common Epithelial Ovarian Carcinomas
- PMID: 37326992
- PMCID: PMC10276312
- DOI: 10.1001/jamanetworkopen.2023.18602
Uptake and Outcomes of Neoadjuvant Chemotherapy Among US Patients With Less Common Epithelial Ovarian Carcinomas
Abstract
Importance: Randomized clinical trials examining the effectiveness of neoadjuvant chemotherapy (NACT) for advanced ovarian cancer predominantly included patients with high-grade serous carcinomas. The use and outcomes of NACT in less common epithelial carcinomas are understudied.
Objective: To investigate the uptake and survival outcomes in treatment with NACT for less common histologic subtypes of epithelial ovarian cancer.
Design, setting, and participants: A retrospective cohort study and systematic literature review with meta-analysis was conducted using the National Cancer Database from 2006 to 2017 and the National Cancer Institute's Surveillance, Epidemiology, and End Results Program from 2006 to 2019. Data analysis was performed from July 2022 to April 2023. The evaluation included patients with stage III to IV ovarian cancer with clear cell, mucinous, or low-grade serous histologic subtypes who received multimodal treatment with surgery and chemotherapy.
Exposures: Exposure assignment per the sequence of treatment: primary debulking surgery (PDS) followed by chemotherapy (PDS group) or NACT followed by interval surgery (NACT group).
Main outcomes and measures: Temporal trends and characteristics of NACT use were assessed using multivariable analysis, and overall survival (OS) was assessed with the inverse probability of treatment weighting propensity score.
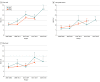
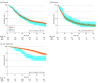
Results: A total of 3880 patients were examined in the National Cancer Database including 1829 women (median age, 56 [IQR, 49-63] years) with clear cell, 1156 women (median age, 53 [IQR, 42-64] years) with low-grade serous, and 895 women (median age, 57 [IQR, 48-66] years) with mucinous carcinomas. NACT use increased in patients with clear cell (from 10.2% to 16.2%, 58.8% relative increase; P < .001 for trend) or low-grade serous (from 7.7% to 14.2%, 84.4% relative increase; P = .007 for trend) carcinoma during the study period. This association remained consistent in multivariable analysis. NACT use also increased, but nonsignificantly, in mucinous carcinomas (from 8.6% to 13.9%, 61.6% relative increase; P = .07 for trend). Across the 3 histologic subtypes, older age and stage IV disease were independently associated with NACT use. In a propensity score-weighted model, the NACT and PDS groups had comparable OS for clear cell (4-year rates, 31.4% vs 37.7%; hazard ratio [HR], 1.12; 95% CI, 0.95-1.33) and mucinous (27.0% vs 26.7%; HR, 0.90; 95% CI, 0.68-1.19) carcinomas. For patients with low-grade serous carcinoma, NACT was associated with decreased OS compared with PDS (4-year rates, 56.4% vs 81.0%; HR, 2.12; 95% CI, 1.55-2.90). Increasing NACT use and histologic subtype-specific survival association were also found in the Surveillance, Epidemiology, and End Results Program cohort (n = 1447). A meta-analysis of 4 studies, including the current study, observed similar OS associations for clear cell (HR, 1.13; 95% CI, 0.96-1.34; 2 studies), mucinous (HR, 0.93; 95% CI, 0.71-1.21; 2 studies), and low-grade serous (HR, 2.11; 95% CI, 1.63-2.74; 3 studies) carcinomas.
Conclusions and relevance: Despite the lack of data on outcomes of NACT among patients with less common carcinomas, this study noted that NACT use for advanced disease has gradually increased in the US. Primary chemotherapy for advanced-stage, low-grade serous ovarian cancer may be associated with worse survival compared with PDS.
Conflict of interest statement
Figures


Similar articles
-
Possible candidate population for neoadjuvant chemotherapy in women with advanced ovarian cancer.Gynecol Oncol. 2021 Jan;160(1):32-39. doi: 10.1016/j.ygyno.2020.10.027. Epub 2020 Oct 24. Gynecol Oncol. 2021. PMID: 33196436
-
Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2021 Jul 30;7(7):CD005343. doi: 10.1002/14651858.CD005343.pub6. Cochrane Database Syst Rev. 2021. PMID: 34328210 Free PMC article.
-
Associations between residual disease and survival in epithelial ovarian cancer by histologic type.Gynecol Oncol. 2017 Nov;147(2):250-256. doi: 10.1016/j.ygyno.2017.08.003. Epub 2017 Aug 16. Gynecol Oncol. 2017. PMID: 28822556
-
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD005343. doi: 10.1002/14651858.CD005343.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2019 Oct 31;2019(10). doi: 10.1002/14651858.CD005343.pub4 PMID: 22895947 Free PMC article. Updated. Review.
-
Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis.Pathology. 2011 Aug;43(5):420-32. doi: 10.1097/PAT.0b013e328348a6e7. Pathology. 2011. PMID: 21716157 Review.
Cited by
-
Exploring novel approaches in the systemic therapy of low-grade serous carcinoma of the ovary: a literature review.Front Med (Lausanne). 2024 May 21;11:1366603. doi: 10.3389/fmed.2024.1366603. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38835797 Free PMC article. Review.
-
Systematic Review of the Survival Outcomes of Neoadjuvant Chemotherapy in Women with Malignant Ovarian Germ Cell Tumors.Cancers (Basel). 2023 Sep 8;15(18):4470. doi: 10.3390/cancers15184470. Cancers (Basel). 2023. PMID: 37760440 Free PMC article. Review.
-
Primary Cytoreduction and Survival for Patients With Less-Common Epithelial Ovarian Cancer.JAMA Netw Open. 2024 Jun 3;7(6):e2417775. doi: 10.1001/jamanetworkopen.2024.17775. JAMA Netw Open. 2024. PMID: 38900429 Free PMC article.
-
Obstetric Characteristics and Outcomes of Gestational Carrier Pregnancies: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2024 Jul 1;7(7):e2422634. doi: 10.1001/jamanetworkopen.2024.22634. JAMA Netw Open. 2024. PMID: 39042408 Free PMC article.
-
Novel Targeted Agents in Advanced and Recurrent Low-Grade Serous Ovarian Cancer: A Silver Lining in the Therapy of a Chemoresistant Disease?Cancers (Basel). 2024 Sep 26;16(19):3268. doi: 10.3390/cancers16193268. Cancers (Basel). 2024. PMID: 39409889 Free PMC article. Review.
References
-
- Coleman RL, Liu JS, Matsuo K, Thaker PH, Westin SN, Sood AK. Carcinoma of the ovaries and fallopian tubes. In: Niederhuber JE, Armitage AO, Doroshow JH, Kastan MB, Tepper JE, eds. Abeloff’s Clinical Oncology. 6th ed. Elsevier; 2019:1525-1543.
-
- National Comprehensive Cancer Network. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. NCCN clinical practice guidelines in Oncology (NCCN Guidelines). Accessed September 30, 2022. https://www.nccn.org/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

