Two Years of Experience and Methodology of Korean COVID-19 Living Clinical Practice Guideline Development
- PMID: 37309700
- PMCID: PMC10261709
- DOI: 10.3346/jkms.2023.38.e195
Two Years of Experience and Methodology of Korean COVID-19 Living Clinical Practice Guideline Development
Abstract
Background: In Korea, during the early phase of the coronavirus disease 2019 (COVID-19) pandemic, we responded to the uncertainty of treatments under various conditions, consistently playing catch up with the speed of evidence updates. Therefore, there was high demand for national-level evidence-based clinical practice guidelines for clinicians in a timely manner. We developed evidence-based and updated living recommendations for clinicians through a transparent development process and multidisciplinary expert collaboration.
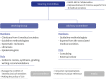
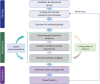
Methods: The National Evidence-based Healthcare Collaborating Agency (NECA) and the Korean Academy of Medical Sciences (KAMS) collaborated to develop trustworthy Korean living guidelines. The NECA-supported methodological sections and 8 professional medical societies of the KAMS worked with clinical experts, and 31 clinicians were involved annually. We developed a total of 35 clinical questions, including medications, respiratory/critical care, pediatric care, emergency care, diagnostic tests, and radiological examinations.
Results: An evidence-based search for treatments began in March 2021 and monthly updates were performed. It was expanded to other areas, and the search interval was organized by a steering committee owing to priority changes. Evidence synthesis and recommendation review was performed by researchers, and living recommendations were updated within 3-4 months.
Conclusion: We provided timely recommendations on living schemes and disseminated them to the public, policymakers and various stakeholders using webpages and social media. Although the output was successful, there were some limitations. The rigor of development issues, urgent timelines for public dissemination, education for new developers, and spread of several new COVID-19 variants have worked as barriers. Therefore, we must prepare systematic processes and funding for future pandemics.
Keywords: COVID-19; GRADE Approach; Living Evidence; Practice Guidelines as Topic; Systematic Review.
© 2023 The Korean Academy of Medical Sciences.
Conflict of interest statement
The COI management committee consisted of 7 members, including the chairs of the GDG, members recommended by the chairs, and outside experts who confirmed the COI declaration form. In the early stage of the practice guidelines development, the COI declaration form was distributed to all committee members (including all members of the GDG) and a survey of the COI was conducted. Throughout the guideline development period, any changes could be voluntarily submitted to the COI management committee. For any reported COI case, at least 2 committee members independently reviewed each case and made determinations. The final decision was made during a general meeting with the COI committee. After the members were reviewed for possible COIs, the COI committee’s decision was held in person or via e-mail. If the COI committee decided that the reported COI impacted at moderate to high levels, members’ activities could be limited to reviewing evidence and not voting for decision-making. Over the 2-year period, 1 member’s reported academic COI was determined as ‘low’ and the other member who reported financial COI was determined as ‘moderate’ by the COI committee (Table 1).
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.Fertil Steril. 2018 Aug;110(3):364-379. doi: 10.1016/j.fertnstert.2018.05.004. Epub 2018 Jul 19. Fertil Steril. 2018. PMID: 30033227 Free PMC article. Review.
-
Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.Hum Reprod. 2018 Sep 1;33(9):1602-1618. doi: 10.1093/humrep/dey256. Hum Reprod. 2018. PMID: 30052961 Free PMC article.
-
Integrating Chinese and western medicine for COVID-19: A living evidence-based guideline (version 1).J Evid Based Med. 2021 Dec;14(4):313-332. doi: 10.1111/jebm.12444. Epub 2021 Oct 11. J Evid Based Med. 2021. PMID: 34632732
-
Methodology for Developing Evidence-Based Clinical Imaging Guidelines: Joint Recommendations by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency.Korean J Radiol. 2017 Jan-Feb;18(1):208-216. doi: 10.3348/kjr.2017.18.1.208. Epub 2017 Jan 5. Korean J Radiol. 2017. PMID: 28096730 Free PMC article. Review.
References
-
- Cheyne S, Fraile Navarro D, Hill K, McDonald S, Tunnicliffe D, White H, et al. Methods for living guidelines: early guidance based on practical experience. J Clin Epidemiol. 2023;155:84–96. - PubMed
-
- Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020;383(18):1757–1766. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical