Design, Synthesis, and Evaluation of a New Series of Hydrazones as Small-Molecule Akt Inhibitors for NSCLC Therapy
- PMID: 37305321
- PMCID: PMC10249096
- DOI: 10.1021/acsomega.3c02331
Design, Synthesis, and Evaluation of a New Series of Hydrazones as Small-Molecule Akt Inhibitors for NSCLC Therapy
Abstract
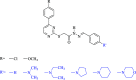
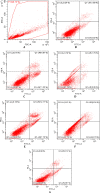
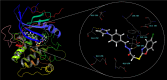
In an endeavor to identify small molecules for the management of non-small-cell lung carcinoma, 10 new hydrazone derivatives (3a-j) were synthesized. MTT test was conducted to examine their cytotoxic activities against human lung adenocarcinoma (A549) and mouse embryonic fibroblast (L929) cells. Compounds 3a, 3e, 3g, and 3i were determined as selective antitumor agents on A549 cell line. Further studies were conducted to figure out their mode of action. Compounds 3a and 3g markedly induced apoptosis in A549 cells. However, both compounds did not show any significant inhibitory effect on Akt. On the other hand, in vitro experiments suggest that compounds 3e and 3i are potential anti-NSCLC agents acting through Akt inhibition. Furthermore, molecular docking studies revealed a unique binding mode for compound 3i (the strongest Akt inhibitor in this series), which interacts with both hinge region and acidic pocket of Akt2. However, it is understood that compounds 3a and 3g exert their cytotoxic and apoptotic effects on A549 cells via different pathway(s).
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
A new series of thiazole-hydrazone hybrids for Akt-targeted therapy of non-small cell lung cancer.Drug Dev Res. 2023 Apr;84(2):185-199. doi: 10.1002/ddr.22022. Epub 2022 Dec 5. Drug Dev Res. 2023. PMID: 36469421
-
A new series of benzoxazole-based SIRT1 modulators for targeted therapy of non-small-cell lung cancer.Arch Pharm (Weinheim). 2021 Jan;354(1):e2000235. doi: 10.1002/ardp.202000235. Epub 2020 Sep 15. Arch Pharm (Weinheim). 2021. PMID: 32930414
-
Hydrazide-hydrazones as Small Molecule Tropomyosin Receptor Kina se A (TRKA) Inhibitors: Synthesis, Anticancer Activities, In silico ADME and Molecular Docking Studies.Med Chem. 2022;19(1):47-63. doi: 10.2174/1573406418666220427105041. Med Chem. 2022. PMID: 35490310
-
Synthesis, biological evaluation, drug-likeness, and in silico screening of novel benzylidene-hydrazone analogues as small molecule anticancer agents.Arch Pharm Res. 2016 Feb;39(2):191-201. doi: 10.1007/s12272-015-0699-z. Epub 2015 Dec 23. Arch Pharm Res. 2016. PMID: 26694484
-
A New Series of Triazolothiadiazines as Potential Anticancer Agents for Targeted Therapy of Non-Small Cell Lung and Colorectal Cancers: Design, Synthesis, In silico and In vitro Studies Providing Mechanistic Insight into Their Anticancer Potencies.Med Chem. 2021;17(10):1104-1128. doi: 10.2174/1573406416666201021142832. Med Chem. 2021. PMID: 33087032
References
LinkOut - more resources
Full Text Sources
Miscellaneous

