The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis
- PMID: 37296616
- PMCID: PMC10253175
- DOI: 10.3390/cells12111495
The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis
Abstract
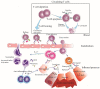
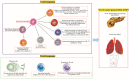
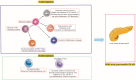
Acute pancreatitis (AP) is an abrupt, variable inflammatory condition of the pancreas, potentially escalating to severe systemic inflammation, rampant pancreatic necrosis, and multi-organ failure. Its complex pathogenesis involves an intricate immune response, with different T cell subsets (Th1, Th2, Th9, Th17, Th22, TFH, Treg, and CD8+ T cells) and B cells playing pivotal roles. Early T cell activation initiates the AP development, triggering cytokines associated with the Th1 response, which stimulate macrophages and neutrophils. Other T cell phenotypes contribute to AP's pathogenesis, and the balance between pro-inflammatory and anti-inflammatory cytokines influences its progression. Regulatory T and B cells are crucial for moderating the inflammatory response and promoting immune tolerance. B cells further contribute through antibody production, antigen presentation, and cytokine secretion. Understanding these immune cells' roles in AP could aid in developing new immunotherapies to enhance patient outcomes. However, further research is required to define these cells' precise roles in AP and their potential as therapeutic targets.
Keywords: B cells; T cells; acute pancreatitis; immune response.
Conflict of interest statement
The authors declare that they have no competing interest or conflict of interest with regard to the research, authorship, and publication of this study.
Figures
Similar articles
-
T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells.Front Immunol. 2020 Aug 18;11:2025. doi: 10.3389/fimmu.2020.02025. eCollection 2020. Front Immunol. 2020. PMID: 32973809 Free PMC article. Review.
-
NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis.Gastroenterology. 2020 Jan;158(1):253-269.e14. doi: 10.1053/j.gastro.2019.09.040. Epub 2019 Oct 5. Gastroenterology. 2020. PMID: 31593700
-
Experimental pancreatitis is characterized by rapid T cell activation, Th2 differentiation that parallels disease severity, and improvement after CD4+ T cell depletion.Pancreatology. 2020 Dec;20(8):1637-1647. doi: 10.1016/j.pan.2020.10.044. Epub 2020 Oct 19. Pancreatology. 2020. PMID: 33097430
-
[Alterations of lymphocyte subsets and indicators of immune suppression in patients with acute pancreatitis].Eksp Klin Gastroenterol. 2014;(9):21-5. Eksp Klin Gastroenterol. 2014. PMID: 25916128 Russian.
-
The immune potential and immunopathology of cytokine-producing B cell subsets: a comprehensive review.J Autoimmun. 2014 Dec;55:10-23. doi: 10.1016/j.jaut.2014.04.001. Epub 2014 May 1. J Autoimmun. 2014. PMID: 24794622 Review.
Cited by
-
Identification and analysis of chemokine-related and NETosis-related genes in acute pancreatitis to develop a predictive model.Front Genet. 2024 May 9;15:1389936. doi: 10.3389/fgene.2024.1389936. eCollection 2024. Front Genet. 2024. PMID: 38784040 Free PMC article.
-
Intestinal Mucosal Immune Barrier: A Powerful Firewall Against Severe Acute Pancreatitis-Associated Acute Lung Injury via the Gut-Lung Axis.J Inflamm Res. 2024 Apr 10;17:2173-2193. doi: 10.2147/JIR.S448819. eCollection 2024. J Inflamm Res. 2024. PMID: 38617383 Free PMC article. Review.
-
Genetic insights into across pancreatitis types: the causal influence of immunoglobulin G N-glycosylation variants on disease risk.Front Immunol. 2024 Mar 18;15:1326370. doi: 10.3389/fimmu.2024.1326370. eCollection 2024. Front Immunol. 2024. PMID: 38566993 Free PMC article.
-
Nicotinamide adenine dinucleotide phosphate oxidase in pancreatic diseases: Mechanisms and future perspectives.World J Gastroenterol. 2024 Feb 7;30(5):429-439. doi: 10.3748/wjg.v30.i5.429. World J Gastroenterol. 2024. PMID: 38414585 Free PMC article. Review.
-
Identification of novel biomarkers based on lipid metabolism-related molecular subtypes for moderately severe and severe acute pancreatitis.Lipids Health Dis. 2024 Jan 2;23(1):1. doi: 10.1186/s12944-023-01972-3. Lipids Health Dis. 2024. PMID: 38169383 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

