CD22 CAR T-cell associated hematologic toxicities, endothelial activation and relationship to neurotoxicity
- PMID: 37295816
- PMCID: PMC10277551
- DOI: 10.1136/jitc-2022-005898
CD22 CAR T-cell associated hematologic toxicities, endothelial activation and relationship to neurotoxicity
Abstract
Background: Hematologic toxicities, including coagulopathy, endothelial activation, and cytopenias, with CD19-targeted chimeric antigen receptor (CAR) T-cell therapies correlate with cytokine release syndrome (CRS) and neurotoxicity severity, but little is known about the extended toxicity profiles of CAR T-cells targeting alternative antigens. This report characterizes hematologic toxicities seen following CD22 CAR T-cells and their relationship to CRS and neurotoxicity.
Methods: We retrospectively characterized hematologic toxicities associated with CRS seen on a phase 1 study of anti-CD22 CAR T-cells for children and young adults with relapsed/refractory CD22+ hematologic malignancies. Additional analyses included correlation of hematologic toxicities with neurotoxicity and exploring effects of hemophagocytic lymphohistiocytosis-like toxicities (HLH) on bone marrow recovery and cytopenias. Coagulopathy was defined as evidence of bleeding or abnormal coagulation parameters. Hematologic toxicities were graded by Common Terminology Criteria for Adverse Events V.4.0.
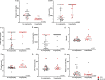
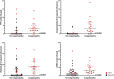
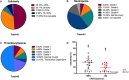
Results: Across 53 patients receiving CD22 CAR T-cells who experienced CRS, 43 (81.1%) patients achieved complete remission. Eighteen (34.0%) patients experienced coagulopathy, of whom 16 had clinical manifestations of mild bleeding (typically mucosal bleeding) which generally subsided following CRS resolution. Three had manifestations of thrombotic microangiopathy. Patients with coagulopathy had higher peak ferritin, D-dimer, prothrombin time, international normalized ratio (INR), lactate dehydrogenase (LDH), tissue factor, prothrombin fragment F1+2 and soluble vascular cell adhesion molecule-1 (s-VCAM-1). Despite a relatively higher incidence of HLH-like toxicities and endothelial activation, overall neurotoxicity was generally less severe than reported with CD19 CAR T-cells, prompting additional analysis to explore CD22 expression in the central nervous system (CNS). Single-cell analysis revealed that in contrast to CD19 expression, CD22 is not on oligodendrocyte precursor cells or on neurovascular cells but is seen on mature oligodendrocytes. Lastly, among those attaining CR, grade 3-4 neutropenia and thrombocytopenia were seen in 65% of patients at D28.
Conclusion: With rising incidence of CD19 negative relapse, CD22 CAR T-cells are increasingly important for the treatment of B-cell malignancies. In characterizing hematologic toxicities on CD22 CAR T-cells, we demonstrate that despite endothelial activation, coagulopathy, and cytopenias, neurotoxicity was relatively mild and that CD22 and CD19 expression in the CNS differed, providing one potential hypothesis for divergent neurotoxicity profiles. Systematic characterization of on-target off-tumor toxicities of novel CAR T-cell constructs will be vital as new antigens are targeted.
Trial registration number: NCT02315612.
Keywords: cytokines; inflammation; receptors, chimeric antigen.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study.Lancet Oncol. 2023 Nov;24(11):1229-1241. doi: 10.1016/S1470-2045(23)00436-9. Epub 2023 Oct 17. Lancet Oncol. 2023. PMID: 37863088 Clinical Trial.
-
Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy.Blood Adv. 2022 Apr 12;6(7):2055-2068. doi: 10.1182/bloodadvances.2020004142. Blood Adv. 2022. PMID: 34666344 Free PMC article.
-
Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells.Blood. 2021 Dec 16;138(24):2469-2484. doi: 10.1182/blood.2021011898. Blood. 2021. PMID: 34525183 Free PMC article. Clinical Trial.
-
Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management.Blood Rev. 2019 Mar;34:45-55. doi: 10.1016/j.blre.2018.11.002. Epub 2018 Nov 14. Blood Rev. 2019. PMID: 30528964 Free PMC article. Review.
-
Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy.Curr Res Transl Med. 2018 May;66(2):50-52. doi: 10.1016/j.retram.2018.03.003. Epub 2018 Apr 3. Curr Res Transl Med. 2018. PMID: 29625831 Free PMC article. Review.
Cited by
-
Novel and multiple targets for chimeric antigen receptor-based therapies in lymphoma.Front Oncol. 2024 Apr 22;14:1396395. doi: 10.3389/fonc.2024.1396395. eCollection 2024. Front Oncol. 2024. PMID: 38711850 Free PMC article. Review.
-
The Mechanisms of Altered Blood-Brain Barrier Permeability in CD19 CAR T-Cell Recipients.Int J Mol Sci. 2024 Jan 4;25(1):644. doi: 10.3390/ijms25010644. Int J Mol Sci. 2024. PMID: 38203814 Free PMC article. Review.
-
Endothelial activation and damage as a common pathological substrate in different pathologies and cell therapy complications.Front Med (Lausanne). 2023 Nov 14;10:1285898. doi: 10.3389/fmed.2023.1285898. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38034541 Free PMC article. Review.
-
CAR-T Cell Therapy in the Treatment of Pediatric Non-Hodgkin Lymphoma.J Pers Med. 2023 Nov 10;13(11):1595. doi: 10.3390/jpm13111595. J Pers Med. 2023. PMID: 38003910 Free PMC article. Review.
-
Immune Effector Cell-Associated HLH-like Syndrome: A Review of the Literature of an Increasingly Recognized Entity.Cancers (Basel). 2023 Oct 26;15(21):5149. doi: 10.3390/cancers15215149. Cancers (Basel). 2023. PMID: 37958323 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
