Photoenhanced Radical Formation in Aqueous Mixtures of Levoglucosan and Benzoquinone: Implications to Photochemical Aging of Biomass-Burning Organic Aerosols
- PMID: 37285129
- PMCID: PMC10291547
- DOI: 10.1021/acs.jpca.3c01794
Photoenhanced Radical Formation in Aqueous Mixtures of Levoglucosan and Benzoquinone: Implications to Photochemical Aging of Biomass-Burning Organic Aerosols
Abstract
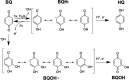
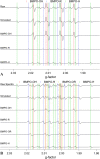
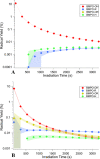
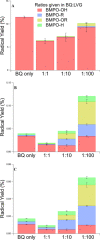
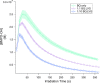
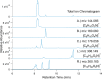
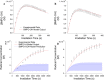
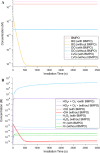
The photochemical aging of biomass-burning organic aerosols (BBOAs) by exposure to sunlight changes the chemical composition over its atmospheric lifetime, affecting the toxicological and climate-relevant properties of BBOA particles. This study used electron paramagnetic resonance (EPR) spectroscopy with a spin-trapping agent, 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO), high-resolution mass spectrometry, and kinetic modeling to study the photosensitized formation of reactive oxygen species (ROS) and free radicals in mixtures of benzoquinone and levoglucosan, known BBOA tracer molecules. EPR analysis of irradiated benzoquinone solutions showed dominant formation of hydroxyl radicals (•OH), which are known products of reaction of triplet-state benzoquinone with water, also yielding semiquinone radicals. In addition, hydrogen radicals (H•) were also observed, which were not detected in previous studies. They were most likely generated by photochemical decomposition of semiquinone radicals. The irradiation of mixtures of benzoquinone and levoglucosan led to substantial formation of carbon- and oxygen-centered organic radicals, which became prominent in mixtures with a higher fraction of levoglucosan. High-resolution mass spectrometry permitted direct observation of BMPO-radical adducts and demonstrated the formation of •OH, semiquinone radicals, and organic radicals derived from oxidation of benzoquinone and levoglucosan. Mass spectrometry also detected superoxide radical adducts (BMPO-OOH) that did not appear in the EPR spectra. Kinetic modeling of the processes in the irradiated mixtures successfully reproduced the time evolution of the observed formation of the BMPO adducts of •OH and H• observed with EPR. The model was then applied to describe photochemical processes that would occur in mixtures of benzoquinone and levoglucosan in the absence of BMPO, predicting the generation of HO2• due to the reaction of H• with dissolved oxygen. These results imply that photoirradiation of aerosols containing photosensitizers induces ROS formation and secondary radical chemistry to drive photochemical aging of BBOA in the atmosphere.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Chemical and Cellular Formation of Reactive Oxygen Species from Secondary Organic Aerosols in Epithelial Lining Fluid.Res Rep Health Eff Inst. 2023 Dec;2023(215):1-56. Res Rep Health Eff Inst. 2023. PMID: 38420854 Free PMC article.
-
Reactive Oxygen Species Formation and Peroxide and Carbonyl Decomposition in Aqueous Photolysis of Secondary Organic Aerosols.Environ Sci Technol. 2024 Mar 12;58(10):4716-4726. doi: 10.1021/acs.est.3c08662. Epub 2024 Feb 27. Environ Sci Technol. 2024. PMID: 38412378
-
EPR Study of KO2 as a Source of Superoxide and •BMPO-OH/OOH Radical That Cleaves Plasmid DNA and Detects Radical Interaction with H2S and Se-Derivatives.Antioxidants (Basel). 2021 Aug 13;10(8):1286. doi: 10.3390/antiox10081286. Antioxidants (Basel). 2021. PMID: 34439533 Free PMC article.
-
Detection and characterisation of radicals in biological materials using EPR methodology.Biochim Biophys Acta. 2014 Feb;1840(2):708-21. doi: 10.1016/j.bbagen.2013.03.034. Epub 2013 Apr 6. Biochim Biophys Acta. 2014. PMID: 23567797 Review.
-
15N-Labeled 4-oxo-2,2,6,6-tetramethyl-piperidine-1-oxyl.2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Apr 30 [updated 2008 Jun 9]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641553 Free Books & Documents. Review.
References
-
- Liu D.; He C.; Schwarz J. P.; Wang X. Lifecycle of Light-Absorbing Carbonaceous Aerosols in the Atmosphere. npj Clim. Atmos. Sci. 2020, 3, 40.10.1038/s41612-020-00145-8. - DOI
-
- Hodshire A. L.; Akherati A.; Alvarado M. J.; Brown-Steiner B.; Jathar S. H.; Jimenez J. L.; Kreidenweis S. M.; Lonsdale C. R.; Onasch T. B.; Ortega A. M.; et al. Aging Effects on Biomass Burning Aerosol Mass and Composition: A Critical Review of Field and Laboratory Studies. Environ. Sci. Technol. 2019, 53, 10007–10022. 10.1021/acs.est.9b02588. - DOI - PubMed
-
- Fang Z.; Li C.; He Q.; Czech H.; Gröger T.; Zeng J.; Fang H.; Xiao S.; Pardo M.; Hartner E.; et al. Secondary Organic Aerosols Produced from Photochemical Oxidation of Secondarily Evaporated Biomass Burning Organic Gases: Chemical Composition, Toxicity, Optical Properties, and Climate Effect. Environ. Int. 2021, 157, 106801.10.1016/j.envint.2021.106801. - DOI - PubMed
-
- Forrister H.; Liu J.; Scheuer E.; Dibb J.; Ziemba L.; Thornhill K. L.; Anderson B.; Diskin G.; Perring A. E.; Schwarz J. P.; et al. Evolution of Brown Carbon in Wildfire Plumes. Geophys. Res. Lett. 2015, 42, 4623–4630. 10.1002/2015GL063897. - DOI

