Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease
- PMID: 37276335
- PMCID: PMC10241721
- DOI: 10.1002/14651858.CD012066.pub3
Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease
Abstract
Background: Long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and inhaled corticosteroids (ICSs) are inhaled medications used to manage chronic obstructive pulmonary disease (COPD). When two classes of medications are required, a LAMA plus an ICS (LABA+ICS) were previously recommended within a single inhaler as the first-line treatment for managing stable COPD in people in high-risk categories. However, updated international guidance recommends a LAMA plus a LABA (LAMA+LABA). This systematic review is an update of a Cochrane Review first published in 2017.
Objectives: To compare the benefits and harms of LAMA+LABA versus LABA+ICS for treatment of people with stable COPD.
Search methods: We performed an electronic search of the Cochrane Airways Group Specialised Register, ClinicalTrials.gov, and the World Health Organization Clinical Trials Search Portal, followed by handsearches. Two review authors screened the selected articles. The most recent search was run on 10 September 2022.
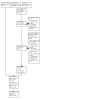
Selection criteria: We included parallel or cross-over randomised controlled trials of at least one month's duration, comparing LAMA+LABA and LABA+ICS for stable COPD. We included studies conducted in an outpatient setting and irrespective of blinding.
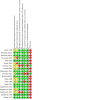
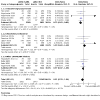
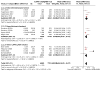
Data collection and analysis: Two review authors independently extracted data and evaluated risk of bias. We resolved any discrepancies through discussion. We analysed dichotomous data as odds ratios (ORs), and continuous data as mean differences (MDs), with 95% confidence intervals (CIs) using Review Manager 5. Primary outcomes were: participants with one or more exacerbations of COPD; serious adverse events; quality of life, as measured by the St. George's Respiratory Questionnaire (SGRQ) total score change from baseline; and trough forced expiratory volume in one second (FEV1). We used the GRADE framework to rate our certainty of the evidence in each meta-analysis as high, moderate, low or very low. MAIN RESULTS: This review updates the first version of the review, published in 2017, and increases the number of included studies from 11 to 19 (22,354 participants). The median number of participants per study was 700. In each study, between 54% and 91% (median 70%) of participants were males. Study participants had an average age of 64 years and percentage predicted FEV1 of 51.5% (medians of study means). Included studies had a generally low risk of selection, performance, detection, attrition, and reporting biases. All but two studies were sponsored by pharmaceutical companies, which had varying levels of involvement in study design, conduct, and data analysis. Primary outcomes The odds of having an exacerbation were similar for LAMA+LABA compared with LABA+ICS (OR 0.91, 95% CI 0.78 to 1.06; I2 = 61%; 13 studies, 20,960 participants; moderate-certainty evidence). The odds of having a serious adverse event were also similar (OR 1.02, 95% CI 0.91 to 1.15; I2 = 20%; 18 studies, 23,183 participants; high-certainty evidence). Participants receiving LAMA+LABA had a similar improvement in quality of life, as measured by the SGRQ, to those receiving LABA+ICS (MD -0.57, 95% CI -1.36 to 0.21; I2 = 78%; 9 studies, 14,437 participants; moderate-certainty evidence) but showed a greater improvement in trough FEV1 (MD 0.07, 95% CI 0.05 to 0.08; I2 = 73%; 12 studies, 14,681 participants; moderate-certainty evidence). Secondary outcomes LAMA+LABA decreased the odds of pneumonia compared with LABA+ICS from 5% to 3% (OR 0.61, 95% CI 0.52 to 0.72; I2 = 0%; 14 studies, 21,829 participants; high-certainty evidence) but increased the odds of all-cause death from 1% to 1.4% (OR 1.35, 95% CI 1.05 to 1.75; I2 = 0%; 15 studies, 21,510 participants; moderate-certainty evidence). The odds of achieving a minimal clinically important difference of four or more points on the SGRQ were similar between LAMA+LABA and LABA+ICS (OR 1.06, 95% CI 0.90 to 1.25; I2 = 77%; 4 studies, 13,614 participants; moderate-certainty evidence).
Authors' conclusions: Combination LAMA+LABA therapy probably holds similar benefits to LABA+ICS for exacerbations and quality of life, as measured by the St George's Respiratory Questionnaire, for people with moderate to severe COPD, but offers a larger improvement in FEV1 and a slightly lower risk of pneumonia. There is little to no difference between LAMA+LABA and LAMA+ICS in the odds of having a serious adverse event. Whilst all-cause death may be lower with LABA+ICS, there was a very small number of events in the analysis, translating to a low absolute risk. Findings are based on moderate- to high-certainty evidence from heterogeneous trials with an observation period of less than one year. This review should be updated again in a few years.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
NF: none known. NH: personally received a lecture fee from AstraZeneca. AK: none known. AG: none known. TK: acted as an independent contractor, with monies paid to institution, for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Novartis. TK also received grants with monies paid to host institution from Boehringer Ingelheim and Novartis. EO: none known. KK: worked as a freelance editor with Cochrane Airways at the time of writing but was not involved in the editorial process for this review.
Figures









Update of
-
Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2017 Feb 10;2(2):CD012066. doi: 10.1002/14651858.CD012066.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 5;6:CD012066. doi: 10.1002/14651858.CD012066.pub3 PMID: 28185242 Free PMC article. Updated. Review.
Similar articles
-
Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2017 Feb 10;2(2):CD012066. doi: 10.1002/14651858.CD012066.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 5;6:CD012066. doi: 10.1002/14651858.CD012066.pub3 PMID: 28185242 Free PMC article. Updated. Review.
-
Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2019 Mar 6;3(3):CD012930. doi: 10.1002/14651858.CD012930.pub2. Cochrane Database Syst Rev. 2019. PMID: 30839102 Free PMC article.
-
Once-daily long-acting beta₂-agonists/inhaled corticosteroids combined inhalers versus inhaled long-acting muscarinic antagonists for people with chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2018 Aug 24;8(8):CD012355. doi: 10.1002/14651858.CD012355.pub2. Cochrane Database Syst Rev. 2018. PMID: 30141826 Free PMC article. Review.
-
Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis.Cochrane Database Syst Rev. 2014 Mar 26;2014(3):CD010844. doi: 10.1002/14651858.CD010844.pub2. Cochrane Database Syst Rev. 2014. PMID: 24671923 Free PMC article. Review.
-
Combined aclidinium bromide and long-acting beta2-agonist for chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2018 Dec 11;12(12):CD011594. doi: 10.1002/14651858.CD011594.pub2. Cochrane Database Syst Rev. 2018. PMID: 30536566 Free PMC article.
Cited by
-
An Evidence-Based Update on Anticholinergic Use for Drug-Induced Movement Disorders.CNS Drugs. 2024 Apr;38(4):239-254. doi: 10.1007/s40263-024-01078-z. Epub 2024 Mar 19. CNS Drugs. 2024. PMID: 38502289 Free PMC article. Review.
-
Inhaled corticosteroid treatment and pneumonia in patients with chronic obstructive pulmonary disease - nationwide development from 1998 to 2018.Eur Clin Respir J. 2024 May 29;11(1):2359768. doi: 10.1080/20018525.2024.2359768. eCollection 2024. Eur Clin Respir J. 2024. PMID: 38817947 Free PMC article.
-
Impact of exacerbation history on future risk and treatment outcomes in chronic obstructive pulmonary disease patients: A prospective cohort study based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) A and B classifications.J Glob Health. 2024 Oct 11;14:04202. doi: 10.7189/jogh.14.04202. J Glob Health. 2024. PMID: 39388682 Free PMC article.
References
References to studies included in this review
Beeh 2016 {published data only}
-
- Beeh KM, Derom E, Echave-Sustaeta J, Grönke L, Hamilton A, Zhai D, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler (ENERGITO study). International Journal of Chronic Obstructive Pulmonary Disease 2016;11:193-205. [DOI: 10.2147/COPD.S95055] [PMID: ] - DOI - PMC - PubMed
-
- Derom E, Beeh K, Echave-Sustaeta J, Grönke L, Zhai D, Bjermer L. Tiotropium + olodaterol provides significant lung-function benefits compared to fluticasone + salmeterol regardless of prior bronchodilator use. European Respiratory Journal 2016;48:PA978. [DOI: 10.1183/13993003.congress-2016] - DOI
Donohue 2015a {published data only (unpublished sought but not used)}
-
- Donohue J, Worsley S, Zhu CQ, Hardaker L, Church A. Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate-to-severe COPD and infrequent COPD exacerbations. Chest 2014;146(4):73A.
-
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870-81. [DOI: 10.1016/j.rmed.2015.04.018] [PMID: ] - DOI - PubMed
Donohue 2015b {published data only (unpublished sought but not used)}
-
- Donohue J, Worsley S, Zhu CQ, Hardaker L, Church A. Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate-to-severe COPD and infrequent COPD exacerbations. Chest 2014;146(4):73A.
-
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870-81. [DOI: 10.1016/j.rmed.2015.04.018] [PMID: ] - DOI - PubMed
Ferguson 2018 {published data only}
-
- Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, et al . Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respiratory Medicine 2018;10:747-58. [DOI: 10.1016/S2213-2600(18)30327-8] [PMID: ] - DOI - PubMed
-
- JPRN-JapicCTI-184079. A Randomized, Double-Blind, Parallel-Group, 24-Week, Chronic-Dosing, Multi-Center Study to Assess the Efficacy and Safety of PT010, PT003, and PT009 Compared With Symbicort Turbuhaler as an Active Control in Subjects With Moderate to Very Severe Chronic Obstructive Pulmonary Disease (PT010006). www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-184079.
-
- JPRN-JapicCTI-184080. A Randomized, Double-Blind, Parallel-Group, 28-Week, Chronic-Dosing, Multi-Center, Extension Study to Assess the Safety and Efficacy of PT010, PT003, and PT009 in Japanese Subjects With Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD) Compared With Symbicort Turbuhaler as an Active Control (PT010007). www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-184080.
-
- NCT03262012. Study to assess the safety and efficacy of PT010, PT003, and PT009 in Japanese subjects with COPD compared with Symbicort Turbohaler. ClinicalTrials.gov/show/NCT03262012 (first posted 25 August 2017).
Frith 2018 {published data only}
-
- Frith PA, Ashmawi S, Krishnamurthy S, Gurgun A, Hristoskova S, Pilipovic V, et al. Efficacy and safety of the direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in non-frequently exacerbating COPD patients: the FLASH randomized controlled trial. Respirology 2018;12:1152-9. [DOI: 10.1111/resp.13374] [PMID: ] - DOI - PubMed
Herth 2020 {published data only}
-
- Herth F, Hohlfeld JM, Haas J, Hoz A, Jin X, Kreitner K-F, et al. The effect of tiotropium/olodaterol versus fluticasone propionate/salmeterol on left ventricular filling and lung hyperinflation in patients with COPD. BMJ Open Respiratory Research 2020;7(1):e000741. [DOI: 10.1136/bmjresp-2020-000741] [PMID: ] - DOI - PMC - PubMed
Hoshino 2015 {published data only}
Lipson 2018 {published data only}
-
- Lipson DA, Barnhart F, Boucot I, Crim C, Brealey N, Criner GJ, et al. Comparison of LAMA/LABA vs ICS/LABA in high risk COPD patients: pre-specified analysis on lung function and health status from the IMPACT trial. European Respiratory Journal 2018;52:PA4385. [DOI: 10.1183/13993003.congress-2018.PA4385] - DOI
-
- Lipson DA, Barnhart F, Boucot I, Crim C, Brealey N, Criner GJ, et al. Exacerbation outcomes with LAMA/LABA and ICS/LABA in high risk COPD patients in the IMPACT trial. European Respiratory Journal 2018;52:PA4384. [DOI: 10.1183/13993003.congress-2018.PA4384] - DOI
-
- Lipson DA, Barnhart F, Boucot I, Crim C, Brealey N, Criner GJ. Comparison of LAMA/LABA vs ICS/LABA in high risk COPD patients: pre-specified analysis on lung function and health status from the IMPACT trial. Pneumologie 2019;73(Suppl 1):[no pagination].
Magnussen 2012 {published data only}
-
- Magnussen H, Maltais F, Schmidt H, Kesten S, Metzdorf N. Comparison of tiotropium+salmeterol vs. fluticasone+salmeterol on lung volumes, exercise tolerance and locus of symptom limitation. American Journal of Respiratory and Critical Care Medicine 2010;181:A4472.
Mostafa 2021 {published data only}
-
- Mostafa TM, El-Azab GA, Atia GA, Lotfy NS. The effectiveness of 3 combined therapeutic regimens in Egyptian patients with moderate-to-severe chronic obstructive pulmonary disease: a randomized double-blind prospective pilot study. Current Therapeutic Research Clinical and Experimental 2021;94:100625. [DOI: 10.1016/j.curtheres.2021.100625] [PMID: ] - DOI - PMC - PubMed
NCT03240575 {published data only}
-
- NCT03240575. The ENERGITO 2 study compares 2 inhaled medicines for chronic obstructive pulmonary disease (COPD). One medicine is a combination of tiotropium and olodaterol (Stiolto) taken using the Respimat inhaler and the other medicine is a combination of fluticasone and salmeterol taken using the Diskus. ClinicalTrials.gov/show/NCT03240575 (first posted 7 August 2017).
Rabe 2008 {published data only (unpublished sought but not used)}
-
- Rabe KF, Timmer W, Sagriotis A, Viel K. Comparison of a combination of tiotropium and formoterol to salmeterol and fluticasone in moderate COPD. In: European Respiratory Society 15th Annual Congress; 2005 Sep 17-20; Copenhagen. 2005.
Rabe 2020 {published data only}
Singh 2015 {published data only (unpublished sought but not used)}
-
- Singh D, Worsley S, Zhu CQ, Hardaker L, Church A. Umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate-to-severe COPD and infrequent COPD exacerbations. European Respiratory Journal 2014;44(Suppl 58):P290.
Vogelmeier 2013 {published data only (unpublished sought but not used)}
-
- Bateman E, Vogelmeier C, Chen H, Banerji D. Comparison of COPD exacerbations with once-daily QVA149 versus twice-daily salmeterol/fluticasone combination: the ILLUMINATE study. American College of Chest Physicians 2014;145(3):409A.
-
- Bateman ED, Vogelmeier C, Pallante J, Bryant H, Alagappan V, D'Andrea P, et al. Once-daily QVA149 demonstrates superior lung function compared to twice-daily salmeterol/fluticasone in all subgroups of COPD patients: the ILLUMINATE study. American Journal of Respiratory and Critical Care Medicine 2013;187:A4273.
-
- Bateman ED, Vogelmeier C, Pallante J, Bryant H, Alagappan V, D'Andrea P, et al. Once-daily QVA149 improves breathlessness and reduces rescue medication use compared to twice-daily salmeterol/fluticasone in patients with COPD: the ILLUMINATE study. American Journal of Respiratory and Critical Care Medicine 2013;187:A2433.
-
- Mezzi K, Pallante J, Alagappan V, Chen H, Banerji D. Once-daily QVA149 demonstrates superior outcomes in COPD patients previously treated with fixed-dose long-acting β2-agonist/inhaled corticosteroid (LABA/ICS): the ILLUMINATE study. Chest 2014;145(3):424A.
-
- Vogelmeier C, Bateman E, Pallante J, Bryant H, Alagappan VD, Andrea P, et al. Once-daily QVA149 provides superior bronchodilation and improves lung function versus twice-daily fluticasone/salmeterol in COPD patients: the ILLUMINATE study. British Thoracic Society Winter Meeting 2012;67:A149, P194.
Vogelmeier 2016 {published data only (unpublished sought but not used)}
-
- Greulich T, Kostikas K, Gaga M, Aalamian-Mattheis M, Lossi NS, Patalano F, et al. Indacaterol/glycopyrronium reduces the risk of clinically important deterioration after direct switch from baseline therapies in patients with moderate COPD: a post hoc analysis of the CRYSTAL study. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:1229-37. [DOI: 10.2147/COPD.S159732] [PMID: ] - DOI - PMC - PubMed
-
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten A, et al. The efficacy and safety of aclidinium/formoterol fixed-dose combination compared with salmeterol/fluticasone in patients with COPD: results from a phase III study. American Journal of Respiratory and Critical Care Medicine 2015;191:A3974.
Vogelmeier 2017 {published data only}
-
- Vogelmeier CF, Gaga M, Aalamian-Mattheis M, Greulich T, Marin JM, Castellani W, et al. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respiratory Research 2018;18(1):140. [DOI: 10.1186/s12931-017-0622-x] [PMID: ] - DOI - PMC - PubMed
Wedzicha 2016 {published data only (unpublished sought but not used)}
-
- Kwaijtaal M, Wedzicha J, Decramer M, Vestbo J, Banerji D. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. Respirology 2014;TP:177. - PubMed
-
- Wedzicha J, Vestbo J, Gallagher N, Banerji D. A novel study design for the comparison between once-daily QVA149 and twice-daily salmeterol/fluticasone on the reduction of COPD exacerbations: the FLAME study. Chest 2014;145(3):408A.
Zhong 2015 {published data only (unpublished sought but not used)}
-
- Zhong N, Wang C, Zhou X, Zhang N, Humphries M, Wang L, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1015-26. [DOI: 10.2147/COPD.S84436] [PMID: ] - DOI - PMC - PubMed
References to studies excluded from this review
Anzueto 2017 {published data only}
-
- Anzueto AR, Vogelmeier CF, Kostikas K, Mezzi K, Fucile S, Bader G, et al. The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:1325-37. [DOI: 10.2147/COPD.S133307] [PMID: ] - DOI - PMC - PubMed
Aziz 2018 {published data only}
Bruhn 2003 {published data only}
-
- Bruhn C. Chronic obstructive pulmonary disease: recommendation of salmeterol in fixed combination. Deutsche Apotheker-Zeitung 2003;143(11):48-51.
Calverley 2007 {published data only}
-
- Calverley P, Stockley R, Seemungal T, Hagan G, Wedzicha J. Adverse events and mortality in the INSPIRE study (Investigating New Standards for Prophylaxis In Reduction of Exacerbations). In: European Respiratory Society 17th Annual Congress; 2007 Sep 16-18; Stockholm. Vol. 30. 2007:125s P847.
EUCTR2015‐002046‐31‐ES {unpublished data only}
-
- EUCTR2015-002046-31-ES. A research study to compare two treatments for treating a respiratory disease known as asthma-COPD overlap syndrome (ACOS) [A randomised, single blind, cross-over study to compare a fixed dose combination of fluticasone propionate / formoterol fumarate (fluticasone /formoterol breath actuated inhaler (BAI)) with a fixed dose combination of indacaterol maleate / glycopyrronium bromide (Ultibro Breezhaler) in subjects with fixed airflow obstruction and elevated eosinophils]. trialsearch.who.int/?TrialID=EUCTR2015-002046-31-ES (first received 3 February 2016).
Knobil 2004a {published data only}
-
- Knobil K, Kalberg C, Merchant K, Emmett A, Cicale M. Maintenance of bronchodilator response for Advair Diskus 250/50 (fluticasone propionate/salmeterol) but not ipratropium/albuterol in patients with COPD. Chest 2004;126(Suppl 4):807S.
Knobil 2004b {published data only}
-
- Knobil K, Merchant K, Kalberg C, Emmett A, Cicale M. A comparison of patient perceived improvement in symptoms after initiating therapy with either Advair Diskus (fluticasone propionate/salmeterol) 250/50 or ipratropium/albuterol. Chest 2004;126(Suppl 4):806S-b-807S-b.
Mahler 2016 {published data only}
Michael 2016 {published data only}
-
- Michael JA, Dana Z. Is combination long-acting beta-agonist and long-acting muscarinic antagonist therapy the future of COPD therapy? Clinical Pulmonary Medicine 2016;23(6):288-9. [DOI: 10.1097/CPM.0000000000000181] - DOI
NCT00120978 {published data only}
-
- NCT00120978. Can advair and flovent reduce systemic inflammation related to chronic obstructive pulmonary disease (COPD)? A multi-center randomized controlled trial. clinicaltrials.gov/ct2/show/NCT00120978 (first received 19 July 2005).
NCT03376295 {unpublished data only}
-
- NCT03376295. Comparative effectiveness of COPD treatments. clinicaltrials.gov/show/NCT03376295 (first posted 18 December 2017).
NCT03504527 {unpublished data only}
-
- NCT03504527. Efficiency of budesonide combined with formoterol and tiotropium in the treatment of acute exacerbation of ACO. ClinicalTrials.gov/show/NCT03504527 (first posted 20 April 2018).
NCT04138758 {unpublished data only}
-
- NCT04138758. Comparative effectiveness and safety of tiotropium and olodaterol in comparison to LABA/ICS [Effectiveness and safety of maintenance treatment with combination of tiotropium and olodaterol in comparison to maintenance treatment with a combination of inhaled corticosteroids and long-acting β2 agonists in COPD patients]. clinicaltrials.gov/ct2/show/NCT04138758 (first posted 30 November 2020).
NCT04320342 {unpublished data only}
-
- NCT04320342. A Phase III Study Comparing Efficacy, Safety and Tolerability of the Fixed Dose Triple Combination CHF 5993 With the Fixed Dose Dual Combination CHF 1535 in Subjects With COPD. clinicaltrials.gov/show/NCT04320342 (first posted 25 March 2020).
NCT04923347 {unpublished data only}
-
- NCT04923347. A Study to Evaluate the Safety and Efficacy of Fluticasone Furoate (FF)/Umeclidinium(UMEC)/Vilanterol (VI) in Participants With Chronic Obstructive Pulmonary Disease (COPD). clinicaltrials.gov/ct2/show/NCT04923347 (first posted 11 June 2021).
NCT05097014 {unpublished data only}
-
- NCT05097014. CHF5993 and CHF1535 pMDI on Lung Hyperinflation and Exercise Endurance Time in Subjects With COPD. clinicaltrials.gov/show/NCT05097014 (first posted 27 October 2021).
Oba 2016 {published data only}
Papi 2018 {published data only}
-
- Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 2018;391(10125):1076-84. [DOI: 10.1016/S0140-6736(18)30206-X] [PMID: ] - DOI - PubMed
Pavord 2016 {published data only}
Price 2014 {published data only}
-
- Price D, Keininger D, Costa-Scharplatz M, Mezzi K, Dimova M, Asukai Y, et al. Cost-effectiveness of the LABA/LAMA dual bronchodilator indacaterol/glycopyrronium in a Swedish healthcare setting. Respiratory Medicine 2014;108(12):1786-93. - PubMed
Sciurba 2004 {published data only}
-
- Sciurba FC, Kalberg C, Emmett A, Merchant K, Brown C, Knobil K. Efficacy of Advair Diskus 250/50 (fluticasone propionate/salmeterol) or ipratropium/albuterol in patients with COPD associated with chronic bronchitis and/or emphysema. Chest 2004;126(Suppl 4):807S-a-808S-a.
Singh 2019 {published data only}
Skoupa 2018 {published data only}
UMIN000024905 {unpublished data only}
-
- UMIN000024905. A randomized, open-label, crossover study to evaluate ICS/LABA treatment versus LAMA/LABA treatment in COPD with eosinophilic inflammation. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000028656 (first posted 21 November 2016).
References to ongoing studies
EUCTR2016‐004473‐41‐GB {unpublished data only}
-
- EUCTR2016-004473-41-GB. Investigating whether two different COPD inhalers have different effects on chest infections [INvestigating COPD Outcomes, Genomics and Neutrophilic Inflammation with Tiotropium and Olodaterol (INCOGNITO trial) - INCOGNITO trial]. trialsearch.who.int/?TrialID=EUCTR2016-004473-41-GB (first received 21 March 2017).
Additional references
Alagha 2014
Anderson 2014
-
- Anderson R, Theron AJ, Steel HC, Durandt C, Tintinger GR, Feldman C. The beta-2-adrenoreceptor agonists, formoterol and indacaterol, but not salbutamol, effectively suppress the reactivity of human neutrophils in vitro. Mediators of Inflammation 2014;2014:105420. [DOI: 10.1155/2014/105420] [PMID: ] - DOI - PMC - PubMed
Barnes 2010
Burrows 1966
Donohue 2005
Drivenes 2014
Esteban 2018
Festic 2015
Frampton 2014
GOLD 2016
-
- The Global Initiative for Chronic Obstructive Lung Disease. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. www.goldcopd.org/Guidelines/guidelines-resources.html (accessed 7 December 2016).
GOLD 2023
-
- The Global Initiative for Chronic Obstructive Lung Disease. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023. www.goldcopd.org/2023-gold-report-2/ (accessed 15 December 2022).
GRADEpro [Computer program]
-
- GRADEpro GDT. Version 3.2 for Windows. Hamilton (ON): McMaster University, 2008. Available at gradepro.org.
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI: 10.1136/bmj.39489.470347.AD] [PMID: ] - DOI - PMC - PubMed
Hanania 2008
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horita 2015a
Malerba 2014
-
- Malerba M, Morjaria JB, Radaeli A. Differential pharmacology and clinical utility of emerging combination treatments in the management of COPD - role of umeclidinium/vilanterol. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:687-95. [DOI: 10.2147/COPD.S47792] [PMID: ] - DOI - PMC - PubMed
Moher 2009
Nannini 2013
-
- Nannini LJ, Poole P, Milan SJ, Holmes R, Normansell R. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD003794. [DOI: 10.1002/14651858.CD003794.pub4] [PMID: ] - DOI - PMC - PubMed
Oba 2018
-
- Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD012620. [DOI: 10.1002/14651858.CD012620.pub2] [PMID: ] - DOI - PMC - PubMed
Pascoe 2015
-
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respiratory Medicine 2015;3(6):435-42. [DOI: 10.1016/S2213-2600(15)00106-X] [PMID: ] - DOI - PubMed
Pauwels 2001
-
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American Journal of Respiratory and Critical Care Medicine 2001;163(5):1256-76. [DOI: 10.1164/ajrccm.163.5.2101039] [PMID: ] - DOI - PubMed
Reddel 2021
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schachter 2013
Suissa 2009
Suissa 2018
Tashkin 2004
White 2013
WHO 2019
-
- World Health Organization. Global health estimates: life expectancy and leading causes of death and disability. www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed 1 January 2022).
References to other published versions of this review
Horita 2016
-
- Horita N, Goto A, Ota E, Nakashima K, Nagai K, Kaneko T. Long-acting muscarinic antagonist plus long-acting beta agonist versus long-acting beta agonist plus inhaled corticosteroid for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD012066. [DOI: 10.1002/14651858.CD012066] - DOI - PMC - PubMed
Horita 2017
-
- Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, Kaneko T. Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD012066. [DOI: 10.1002/14651858.CD012066.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

