Study of tumor necrosis factor receptor in the inflammatory bowel disease
- PMID: 37274062
- PMCID: PMC10237104
- DOI: 10.3748/wjg.v29.i18.2733
Study of tumor necrosis factor receptor in the inflammatory bowel disease
Abstract
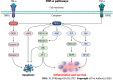
Ulcerative colitis (UC) and Crohn's disease (CD) are part of Inflammatory Bowel Diseases (IBD) and have pathophysiological processes such as bowel necrosis and enteric neurons and enteric glial cells. In addition, the main inflammatory mediator is related to the tumor necrosis factor-alpha (TNF-α). TNF-α is a me-diator of the intestinal inflammatory processes, thus being one of the main cytokines involved in the pathogenesis of IBD, however, its levels, when measured, are present in the serum of patients with IBD. In addition, TNF-α plays an important role in promoting inflammation, such as the production of interleukins (IL), for instance IL-1β and IL-6. There are two receptors for TNF as following: The tumor necrosis factor 1 receptor (TNFR1); and the tumor necrosis factor 2 receptor (TNFR2). They are involved in the pathogenesis of IBD and their receptors have been detected in IBD and their expression is correlated with disease activity. The soluble TNF form binds to the TNFR1 receptor with, and its activation results in a signaling cascade effects such as apoptosis, cell proliferation and cytokine secretion. In contrast, the transmembrane TNF form can bind both to TNFR1 and TNFR2. Recent studies have suggested that TNF-α is one of the main pro-inflammatory cytokines involved in the pathogenesis of IBD, since TNF levels are present in the serum of both patients with UC and CD. Intravenous and subcutaneous biologics targeting TNF-α have revolutionized the treatment of IBD, thus becoming the best available agents to induce and maintain IBD remission. The application of antibodies aimed at neutralizing TNF-α in patients with IBD that induce a satisfactory clinical response in up to 60% of patients, and also induced long-term maintenance of disease remission in most patients. It has been suggested that anti-TNF-α agents inactivate the pro-inflammatory cytokine TNF-α by direct neutralization, i.e., resulting in suppression of inflammation. However, anti-TNF-α antibodies perform more complex functions than a simple blockade.
Keywords: Enteric nervous system; Inflammatory bowel diseases; Tumor necrosis factor 1 receptor; Tumor necrosis factor 2 receptor; tumor necrosis factor-alpha.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Figures


Similar articles
-
Effects of bovine tumor necrosis factor alpha decoy receptors on cell death and inflammatory cytokine kinetics: potential for bovine inflammation therapy.BMC Vet Res. 2019 Feb 28;15(1):68. doi: 10.1186/s12917-019-1813-0. BMC Vet Res. 2019. PMID: 30819151 Free PMC article.
-
Pharmacodynamic mechanisms behind a refractory state in inflammatory bowel disease.BMC Gastroenterol. 2022 Nov 17;22(1):464. doi: 10.1186/s12876-022-02559-5. BMC Gastroenterol. 2022. PMID: 36384462 Free PMC article.
-
TNF-α Autocrine Feedback Loops in Human Monocytes: The Pro- and Anti-Inflammatory Roles of the TNF-α Receptors Support the Concept of Selective TNFR1 Blockade In Vivo.J Immunol Res. 2016;2016:1079851. doi: 10.1155/2016/1079851. Epub 2016 Sep 22. J Immunol Res. 2016. PMID: 27747245 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Management of inflammatory bowel disease beyond tumor necrosis factor inhibitors: novel biologics and small-molecule drugs.Korean J Intern Med. 2022 Sep;37(5):906-919. doi: 10.3904/kjim.2022.152. Epub 2022 Aug 10. Korean J Intern Med. 2022. PMID: 35945034 Free PMC article. Review.
Cited by
-
Navigating new horizons in inflammatory bowel disease: Integrative approaches and innovations.World J Gastroenterol. 2024 Nov 7;30(41):4411-4416. doi: 10.3748/wjg.v30.i41.4411. World J Gastroenterol. 2024. PMID: 39534414 Free PMC article.
-
Ileal inflammation is reduced due to treatment with a metalloprotease from BmooMP-α-I snake venom in an experimental model of Toxoplasma gondii infection.Parasitol Res. 2023 Dec 22;123(1):65. doi: 10.1007/s00436-023-08033-9. Parasitol Res. 2023. PMID: 38133827
-
Advancements in Endoscopic Resection for Colitis-Associated Colorectal Neoplasia in Inflammatory Bowel Disease: Turning Visible into Resectable.Diagnostics (Basel). 2023 Dec 20;14(1):9. doi: 10.3390/diagnostics14010009. Diagnostics (Basel). 2023. PMID: 38201318 Free PMC article. Review.
-
Type 2 autoimmune pancreatitis associated with ulcerative colitis.Front Immunol. 2023 Dec 6;14:1288390. doi: 10.3389/fimmu.2023.1288390. eCollection 2023. Front Immunol. 2023. PMID: 38124742 Free PMC article. Review.
-
Inflammatory Bowel Disease: A Comprehensive Analysis of Molecular Bases, Predictive Biomarkers, Diagnostic Methods, and Therapeutic Options.Int J Mol Sci. 2024 Jun 27;25(13):7062. doi: 10.3390/ijms25137062. Int J Mol Sci. 2024. PMID: 39000169 Free PMC article. Review.
References
-
- Shapiro JM, Subedi S, LeLeiko NS. Inflammatory Bowel Disease. Pediatr Rev . 2016;37:337–347. - PubMed
-
- Shivashankar R, Lichtenstein GR. Mimics of Inflammatory Bowel Disease. Inflamm Bowel Dis . 2018;24:2315–2321. - PubMed
-
- Ahmad T, Satsangi J, McGovern D, Bunce M, Jewell DP. Review article: The genetics of inflammatory bowel disease. Aliment Pharmacol Ther . 2001;15:731–748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

