AGE-RAGE axis culminates into multiple pathogenic processes: a central road to neurodegeneration
- PMID: 37266370
- PMCID: PMC10230046
- DOI: 10.3389/fnmol.2023.1155175
AGE-RAGE axis culminates into multiple pathogenic processes: a central road to neurodegeneration
Abstract
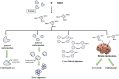
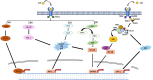
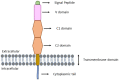
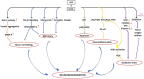
Advanced glycation end-products (AGEs; e.g., glyoxal, methylglyoxal or carboxymethyl-lysine) are heterogenous group of toxic compounds synthesized in the body through both exogenous and endogenous pathways. AGEs are known to covalently modify proteins bringing about loss of functional alteration in the proteins. AGEs also interact with their receptor, receptor for AGE (RAGE) and such interactions influence different biological processes including oxidative stress and apoptosis. Previously, AGE-RAGE axis has long been considered to be the maligning factor for various human diseases including, diabetes, obesity, cardiovascular, aging, etc. Recent developments have revealed the involvement of AGE-RAGE axis in different pathological consequences associated with the onset of neurodegeneration including, disruption of blood brain barrier, neuroinflammation, remodeling of extracellular matrix, dysregulation of polyol pathway and antioxidant enzymes, etc. In the present article, we attempted to describe a new avenue that AGE-RAGE axis culminates to different pathological consequences in brain and therefore, is a central instigating component to several neurodegenerative diseases (NGDs). We also invoke that specific inhibitors of TIR domains of TLR or RAGE receptors are crucial molecules for the therapeutic intervention of NGDs. Clinical perspectives have also been appropriately discussed.
Keywords: AGE; RAGE; Velcade; glycation; neurodegeneration.
Copyright © 2023 Bhattacharya, Alam, Kamal, Seo and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Glycation & the RAGE axis: targeting signal transduction through DIAPH1.Expert Rev Proteomics. 2017 Feb;14(2):147-156. doi: 10.1080/14789450.2017.1271719. Epub 2016 Dec 22. Expert Rev Proteomics. 2017. PMID: 27967251 Free PMC article. Review.
-
Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives.Biomolecules. 2022 Apr 4;12(4):542. doi: 10.3390/biom12040542. Biomolecules. 2022. PMID: 35454131 Free PMC article. Review.
-
[AGEs and RAGE - advanced glycation end-products and their receptor in questions and answers].Vnitr Lek. 2014 Sep;60(9):720-4. Vnitr Lek. 2014. PMID: 25294759 Czech.
-
Advanced glycation endproducts in food and their effects on health.Food Chem Toxicol. 2013 Oct;60:10-37. doi: 10.1016/j.fct.2013.06.052. Epub 2013 Jul 16. Food Chem Toxicol. 2013. PMID: 23867544 Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
Cited by
-
Sodium-glucose cotransporter 1/2 inhibition and risk of neurodegenerative disorders: A Mendelian randomization study.Brain Behav. 2024 Jul;14(7):e3624. doi: 10.1002/brb3.3624. Brain Behav. 2024. PMID: 39010704 Free PMC article.
-
Quantifying carboxymethyl lysine and carboxyethyl lysine in human plasma: clinical insights into aging research using liquid chromatography-tandem mass spectrometry.BMC Biotechnol. 2024 Mar 7;24(1):12. doi: 10.1186/s12896-024-00838-5. BMC Biotechnol. 2024. PMID: 38454400 Free PMC article.
-
Plasma Concentrations of High Mobility Group Box 1 Proteins and Soluble Receptors for Advanced Glycation End-Products Are Relevant Biomarkers of Cognitive Impairment in Alcohol Use Disorder: A Pilot Study.Toxics. 2024 Feb 29;12(3):190. doi: 10.3390/toxics12030190. Toxics. 2024. PMID: 38535924 Free PMC article.
-
Neuroinflammaging: A Tight Line Between Normal Aging and Age-Related Neurodegenerative Disorders.Aging Dis. 2024 Aug 1;15(4):1726-1747. doi: 10.14336/AD.2023.1001. Aging Dis. 2024. PMID: 38300639 Free PMC article. Review.
-
Luteolin targets the AGE-RAGE signaling to mitigate inflammation and ferroptosis in chronic atrophic gastritis.Aging (Albany NY). 2024 Jun 24;16(13):10918-10930. doi: 10.18632/aging.205969. Epub 2024 Jun 24. Aging (Albany NY). 2024. PMID: 38917486 Free PMC article.
References
-
- Akamine T., Kusunose N., Matsunaga N., Koyanagi S., Ohdo S. (2018). Accumulation of sorbitol in the sciatic nerve modulates circadian properties of diabetes-induced neuropathic pain hypersensitivity in a diabetic mouse model. Biochem. Biophys. Res. Commun. 503, 181–187. doi: 10.1016/J.BBRC.2018.05.209, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

