Arsenic binding to human metallothionein-3
- PMID: 37265731
- PMCID: PMC10231319
- DOI: 10.1039/d3sc00400g
Arsenic binding to human metallothionein-3
Abstract
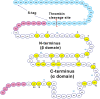
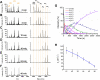
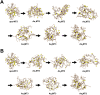
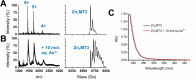
Arsenic poisoning is of great concern with respect to its neurological toxicity, which is especially significant for young children. Human exposure to arsenic occurs worldwide from contaminated drinking water. In human physiology, one response to toxic metals is through coordination with the metallochaperone metallothionein (MT). Central nervous system expression of MT isoform 3 (MT3) is thought to be neuroprotective. We report for the first time on the metalation pathways of As3+ binding to apo-MT3 under physiological conditions, yielding the absolute binding constants (log Kn, n = 1-6) for each sequential As3+ binding event: 10.20, 10.02, 9.79, 9.48, 9.06, and 8.31 M-1. We report on the rate of the reaction of As3+ with apo-MT3 at pH 3.5 with rate constants (kn, n = 1-6) determined for each sequential As3+ binding event: 116.9, 101.2, 85.6, 64.0, 43.9, and 21.0 M-1 s-1. We further characterize the As3+ binding pathway to fully metalated Zn7MT3 and partially metalated Zn-MT3. As3+ binds rapidly with high binding constants under physiological conditions in a noncooperative manner, but is unable to replace the Zn2+ in fully-metalated Zn-MT3. As3+ binding to partially metalated Zn-MT3 takes place with a rearrangement of the Zn-binding profile. Our work shows that As 3+ rapidly and efficiently binds to both apo-MT3 and partially metalated Zn-MT3 at physiological pH.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Metallothionein-3 and carbonic anhydrase metalation properties with Zn(II) and Cd(II) change as a result of protein-protein interactions.Metallomics. 2023 Oct 4;15(10):mfad056. doi: 10.1093/mtomcs/mfad056. Metallomics. 2023. PMID: 37723614
-
Apo-metallothionein-3 cooperatively forms tightly compact structures under physiological conditions.J Biol Chem. 2023 Mar;299(3):102899. doi: 10.1016/j.jbc.2023.102899. Epub 2023 Jan 11. J Biol Chem. 2023. PMID: 36639030 Free PMC article.
-
Noncooperative metalation of metallothionein 1a and its isolated domains with zinc.Biochemistry. 2012 Aug 21;51(33):6690-700. doi: 10.1021/bi3004523. Epub 2012 Aug 7. Biochemistry. 2012. PMID: 22823575
-
Unravelling the mechanistic details of metal binding to mammalian metallothioneins from stoichiometric, kinetic, and binding affinity data.Dalton Trans. 2018 Mar 12;47(11):3613-3637. doi: 10.1039/c7dt03319b. Dalton Trans. 2018. PMID: 29431781 Review.
-
Lead(II) Binding in Metallothioneins.Met Ions Life Sci. 2017 Apr 10;17:/books/9783110434330/9783110434330-009/9783110434330-009.xml. doi: 10.1515/9783110434330-009. Met Ions Life Sci. 2017. PMID: 28731302 Review.
Cited by
-
AsIII Selectively Induces a Disorder-to-Order Transition in the Metalloid Binding Region of the AfArsR Protein.J Am Chem Soc. 2024 Jun 26;146(25):17009-17022. doi: 10.1021/jacs.3c11665. Epub 2024 May 31. J Am Chem Soc. 2024. PMID: 38820242 Free PMC article.
-
ESI-MS analysis of Cu(I) binding to apo and Zn7 human metallothionein 1A, 2, and 3 identifies the formation of a similar series of metallated species with no individual isoform optimization for Cu(I).Metallomics. 2024 Apr 5;16(4):mfae015. doi: 10.1093/mtomcs/mfae015. Metallomics. 2024. PMID: 38503570 Free PMC article.
-
Synthetic bacteria designed using ars operons: a promising solution for arsenic biosensing and bioremediation.World J Microbiol Biotechnol. 2024 May 6;40(6):192. doi: 10.1007/s11274-024-04001-2. World J Microbiol Biotechnol. 2024. PMID: 38709285 Review.
References
-
- IARC, Arsenic, Metals, Fibres, and Dusts, International Agency for Research on Cancer, Lyon, France, 2012
LinkOut - more resources
Full Text Sources
Research Materials

